Goserelin
DRACPC ID DRACPC0002
Active Ingredients Goserelin
Description A synthetic decapeptide analog of luteinizing hormone-releasing hormone (LHRH) with antineoplastic activity. Goserelin binds to and activates pituitary gonadotropin releasing hormone (GnRH) receptors. Prolonged administration of goserelin inhibits the secretion of pituitary gonadotropin, thereby decreasing levels of testosterone (in males) and estradiol (in females). Administration of this agent in a depot formulation may result in the regression of sex hormone-sensitive tumors and a reduction in sex organ size and function.
Synonyms 6-[O-(1,1-Dimethylethyl)-D-serine]-10-deglycinamide Luteinizing Hormone-Releasing Factor (Pig) 2-(aminocarbonyl)hydrazide; Zoladex; Decapeptide I; ICI-118630; Goserelin
Type Small Molecule
Disease Prostate cancer, Breast cancer
Classification
Peptide and derivative Hormone and analogue
Structure Information
Molecular Formula C59H84N18O14
Molecular Weight 1269.433
Active Sequence XHWSYxLRP
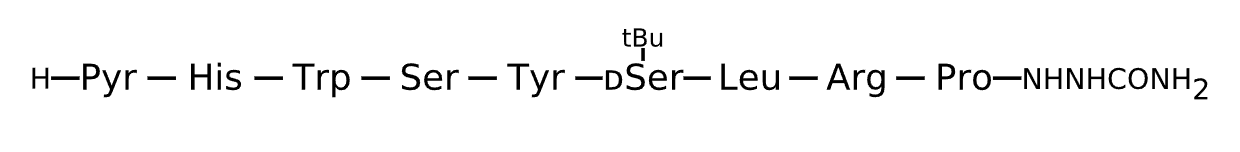
Sequence Length 9
Modification X=Pyr, x=D-Ser(tBu), N-terminal NHNHCONH2
IUPAC Name (2S)-N-[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2R)-1-[[(2S)-1-[[(2S)-1-[(2S)-2-[(carbamoylamino)carbamoyl]pyrrolidin-1-yl]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-[(2-methylpropan-2-yl)oxy]-1-oxopropan-2-yl]amino]-3-(4-hydroxyphenyl)-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-3-(1H-imidazol-5-yl)-1-oxopropan-2-yl]-5-oxopyrrolidine-2-carboxamide
InChI InChI=1S/C64H82N18O13/c1-34(2)23-46(56(88)75-45(13-7-21-69-64(66)67)63(95)82-22-8-14-52(82)62(94)72-31-53(65)85)76-58(90)48(25-36-28-70-42-11-5-3-9-40(36)42)78-57(89)47(24-35-15-17-39(84)18-16-35)77-61(93)51(32-83)81-59(91)49(26-37-29-71-43-12-6-4-10-41(37)43)79-60(92)50(27-38-30-68-33-73-38)80-55(87)44-19-20-54(86)74-44/h3-6,9-12,15-18,28-30,33-34,44-52,70-71,83-84H,7-8,13-14,19-27,31-32H2,1-2H3,(H2,65,85)(H,68,73)(H,72,94)(H,74,86)(H,75,88)(H,76,90)(H,77,93)(H,78,89)(H,79,92)(H,80,87)(H,81,91)(H4,66,67,69)/t44-,45-,46-,47-,48+,49-,50-,51-,52-/m0/s1
InChI_Key VXKHXGOKWPXYNA-PGBVPBMZSA-N
SMILES N=C(N)NCCC[C@H](NC([C@@H](NC([C@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H]1CCC(N1)=O)=O)CC2=CNC=N2)=O)CC3=CNC4=C3C=CC=C4)=O)CO)=O)CC5=CC=C(O)C=C5)=O)CC6=CNC7=CC=CC=C67)=O)CC(C)C)=O)C(N8CCC[C@H]8C(NCC(N)=O)=O)=O
External Codes
PubChem CID 5311128
DrugBank Accession Number DB00014
NCI Thesaurus Code C1374
UNII 0F65R8P09N GSRS
CAS 65807-02-5
Drug approval
Drug indication
Goserelin is indicated for:
1) Use in combination with flutamide for the management of locally confined carcinoma of the prostate
2) Palliative treatment of advanced carcinoma of the prostate
3) The management of endometriosis
4) Use as an endometrial-thinning agent prior to endometrial ablation for dysfunctional uterine bleeding
5) Use in the palliative treatment of advanced breast cancer in pre- and perimenopausal women
| Drug Name | Strength | Dosage Form/Route | Company | Marketing Status | Drug ID | Approval year |
|---|---|---|---|---|---|---|
| Zoladex | eq 3.6mg base | Implant; Implantation | Tersera Theraps Llc | Prescription | NDA: 019726 | 1989 |
| Zoladex | eq 10.8mg base | Implant; Implantation | Tersera Theraps Llc | Prescription | NDA: 020578 | 1996 |
| Zoladex | 3.6 mg | Implant; Subcutaneous | Tersera Therapeutics Llc | Prescription | DIN: 02049325 | 1994 |
| Zoladex | 10.8 mg | Implant; Subcutaneous | Tersera Therapeutics Llc | Prescription | DIN: 02225905 | 1996 |
| ClinicalTrials.gov Identifier | Title | Condition or disease | Phase | Purpose |
|---|---|---|---|---|
| NCT03936218 | A Two Arm, Multi Centric, Randomised, Open Label, Parallel Study to Compare Pharmacodynamics of Subcutaneous Goserelin 10.8mg Injection (Sponsor) With ZOLADEX® 10.8mg Injection (AstraZeneca) in Patients With Advanced Prostate Cancer | Advanced Prostate Cancer | Phase 3 | Treatment |
| NCT00186121 | A Phase II Trial of Arimidex Plus Zoladex in the Treatment of Hormone Receptor Positive, Metastatic Carcinoma of the Breast in Premenopausal Women | Breast Cancer | Phase 2 | Treatment |
| NCT01073865 | An Open Label, Randomised, Parallel Group, Multicentre Study to Compare ZOLADEX™ 10.8 mg Given Every 12 Weeks With ZOLADEX 3.6 mg Given Every 4 Weeks in Pre-menopausal Women With Oestrogen Receptor Positive Advanced Breast Cancer. | Breast Cancer | Phase 3 | Treatment |
| NCT02586675 | The TEEL Study: A Phase I Trial of Tamoxifen With Ribociclib (LEE011) in Adult Patients With Advanced ER+ (HER2 Negative) Breast Cancer | Breast Cancer; Breast Cancer - Female; Breast Cancer - Male | Phase 1 | Treatment |
| NCT02430480 | Neoadjuvant Androgen Deprivation and Enzalutamide: Using Multiparametric MRI to Evaluate Intraprostatic Tumor Responses and Androgen Resistance Patterns in Newly Diagnosed Prostate Cancer | Prostate Cancer | Phase 2 | Treatment |
More clinical information is obtained from ClinicalTrials.gov.