Octreotide
DRACPC ID DRACPC0003
Active Ingredients Octreotide
Description A synthetic long-acting cyclic octapeptide with pharmacologic properties mimicking those of the natural hormone somatostatin. Octreotide is a more potent inhibitor of growth hormone, glucagon, and insulin than somatostatin. Similar to somatostatin, this agent also suppresses the luteinizing hormone response to gonadotropin-releasing hormone, decreases splanchnic blood flow, and inhibits the release of serotonin, gastrin, vasoactive intestinal peptide (VIP), secretin, motilin, pancreatic polypeptide, and thyroid stimulating hormone.
Synonyms SMS-201-995; D-Phenylalanyl-L-cysteinyl-L-phenyl-alanyl-D-tryptophyl-L-lysyl-L-threonyl-N-[2-hydroxy-1-(hydroxymethyl)propyl]-L-cysteinamide Cyclic (2->7)-Disulfide; Octreotide
Type Biotech
Disease Acromegaly, Diarrhea, Metastatic Carcinoid Tumors
Classification
Somatostatin and analogues Peptide and derivative Cyclic
Structure Information
Molecular Formula C49H66N10O10S2
Molecular Weight 1019.247
Active Sequence FCFWKXCT
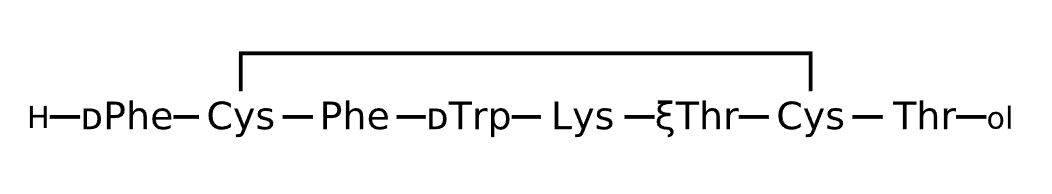
Sequence Length 8
Modification X=xiThr, N-terminal ol
IUPAC Name (4R,7S,10S,13R,16S,19R)-10-(4-aminobutyl)-19-[[(2R)-2-amino-3-phenylpropanoyl]amino]-16-benzyl-N-[(2R,3R)-1,3-dihydroxybutan-2-yl]-7-(1-hydroxyethyl)-13-(1H-indol-3-ylmethyl)-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carboxamide
InChI InChI=1S/C49H66N10O10S2/c1-28(61)39(25-60)56-48(68)41-27-71-70-26-40(57-43(63)34(51)21-30-13-5-3-6-14-30)47(67)54-37(22-31-15-7-4-8-16-31)45(65)55-38(23-32-24-52-35-18-10-9-17-33(32)35)46(66)53-36(19-11-12-20-50)44(64)59-42(29(2)62)49(69)58-41/h3-10,13-18,24,28-29,34,36-42,52,60-62H,11-12,19-23,25-27,50-51H2,1-2H3,(H,53,66)(H,54,67)(H,55,65)(H,56,68)(H,57,63)(H,58,69)(H,59,64)/t28-,29?,34-,36+,37+,38-,39-,40+,41+,42+/m1/s1
InChI_Key DEQANNDTNATYII-RRCPSWKPSA-N
SMILES CC([C@@H]1NC([C@@H](NC([C@H](NC([C@@H](NC([C@H](CSSC[C@H](NC1=O)C(N[C@@H]([C@H](O)C)CO)=O)NC([C@@H](CC2=CC=CC=C2)N)=O)=O)CC3=CC=CC=C3)=O)CC4=CNC5=CC=CC=C45)=O)CCCCN)=O)O
External Codes
PubChem CID 6400441
DrugBank Accession Number DB00104
NCI Thesaurus Code C711
UNII RWM8CCW8GP GSRS
CAS 83150-76-9
Drug approval
Drug indication
Octreotide by injection is used for the treatment of acromegaly and the reduction of flushing and diarrhea symptoms related to carcinoid tumors and/or vasoactive intestinal peptide (VIPoma) tumors. The delayed-release oral formulation is used for the long-term treatment of acromegaly in patients who tolerate and respond adequately to injectable octreotide and lanreotide.
| Drug Name | Strength | Dosage Form/Route | Company | Marketing Status | Drug ID | Approval year |
|---|---|---|---|---|---|---|
| Octreotide Acetate | eq 0.05mg base/ml; eq 0.1mg base/ml; eq 0.5mg base/ml | Injectable; Injection | Teva Pharms Usa | Prescription | ANDA: 075957 | 2005 |
| Octreotide Acetate | eq 0.2mg base/ml; eq 1mg base/ml | Injectable; Injection | Teva Pharms Usa | Prescription | ANDA: 075959 | 2005 |
| Octreotide Acetate | eq 0.2mg base/ml; eq 1mg base/ml | Injectable; Injection | West-Ward Pharms Int | Prescription | ANDA: 076330 | 2005 |
| Octreotide Acetate | eq 0.05mg base/ml; eq 0.1mg base/ml; eq 0.5mg base/ml | Injectable; Injection | Sun Pharm Inds | Discontinued | ANDA: 077329 | 2008 |
| Octreotide Acetate | eq 0.2mg base/ml | Injectable; Injection | Sun Pharm Inds | Discontinued | ANDA: 077330 | 2008 |
| Octreotide Acetate | eq 1mg base/ml | Injectable; Injection | Sun Pharm Inds | Discontinued | ANDA: 077331 | 2008 |
| Octreotide Acetate | eq 0.05mg base/ml; eq 0.1mg base/ml; eq 0.5mg base/ml | Injectable; Injection | Sun Pharm Inds | Prescription | ANDA: 077372 | 2007 |
| Octreotide Acetate | eq 0.2mg base/ml; eq 1mg base/ml | Injectable; Injection | Sun Pharm Inds | Prescription | ANDA: 077373 | 2007 |
| Octreotide Acetate | eq 0.2mg base/ml; eq 1mg base/ml | Injectable; Injection | Fresenius Kabi Usa | Prescription | ANDA: 077450 | 2006 |
| Octreotide Acetate | eq 0.2mg base/ml; eq 1mg base/ml | Injectable; Injection | Wockhardt Usa | Discontinued | ANDA: 090986 | 2011 |
| Octreotide Acetate | eq 0.2mg base/ml; eq 1mg base/ml | Injectable; Injection | Sagent Pharms | Prescription | ANDA: 091041 | 2013 |
| Octreotide Acetate (Preservative Free) | eq 0.05mg base/ml; eq 0.1mg base/ml; eq 0.5mg base/ml | Injectable; Injection | West-Ward Pharms Int | Prescription | ANDA: 076313 | 2005 |
| Octreotide Acetate (Preservative Free) | eq 0.05mg base/ml; eq 0.1mg base/ml; eq 0.5mg base/ml | Injectable; Injection | Fresenius Kabi Usa | Prescription | ANDA: 077457 | 2006 |
| Octreotide Acetate (Preservative Free) | eq 0.05mg base/ml; eq 0.1mg base/ml; eq 0.5mg base/ml | Injectable; Injection | Mylan Institutional | Prescription | ANDA: 079198 | 2011 |
| Octreotide Acetate (Preservative Free) | eq 0.05mg base/ml; eq 0.1mg base/ml; eq 0.5mg base/ml | Injectable; Injection | Sagent Pharms | Prescription | ANDA: 090834 | 2013 |
| Octreotide Acetate Preservative Free | eq 0.05mg base/ml;eq 0.1mg base/ml; eq 0.5mg base/ml | Injectable; Injection | Wockhardt Usa | Discontinued | ANDA: 090985 | 2011 |
| Sandostatin | eq 0.05mg base/ml;eq 0.1mg base/ml; eq 0.5mg base/ml; eq 0.2mg base/ml; eq 1mg base/ml | Injectable; Injection | Novartis | Prescription | NDA: 019667 | 1988 |
| Sandostatin Lar | eq 10mg base/vial; eq 20mg base/vial; eq 30mg base/vial | Injectable; Injection | Novartis | Prescription | NDA: 021008 | 1998 |
| Ocphyl | 50 mcg/ml | Injectable; Injection | Pendopharm Division Of De Pharmascience Inc | Prescription | DIN: 02413191 | 2014 |
| Ocphyl | 100 mcg/ml | Injectable; Injection | Pendopharm Division Of De Pharmascience Inc | Prescription | DIN: 02413205 | 2014 |
| Ocphyl | 500 mcg/ml | Injectable; Injection | Pendopharm Division Of De Pharmascience Inc | Prescription | DIN: 02413213 | 2014 |
| Octreotide Acetate Injection - 100Mcg/Ml | 100 mcg/ml | Injectable; Injection | Sandoz Canada Incorporated | Prescription | DIN: 02263173 | 2005 |
| Octreotide Acetate Injection - 200Mcg/Ml | 200 mcg/ml | Injectable; Injection | Sandoz Canada Incorporated | Prescription | DIN: 02263181 | 2005 |
| Octreotide Acetate Injection - 500Mcg/Ml | 500 mcg/ml | Injectable; Injection | Sandoz Canada Incorporated | Prescription | DIN: 02263203 | 2005 |
| Octreotide Acetate Injection - 50Mcg/Ml | 50 mcg/ml | Injectable; Injection | Sandoz Canada Incorporated | Prescription | DIN: 02263165 | 2005 |
| Octreotide Acetate Omega 100Mcg/Ml | 100 mcg/ml | Injectable; Injection | Omega Laboratories Ltd | Prescription | DIN: 02248640 | 2004 |
| Octreotide Acetate Omega 200Mcg/Ml | 200 mcg/ml | Injectable; Injection | Omega Laboratories Ltd | Prescription | DIN: 02248642 | 2004 |
| Octreotide Acetate Omega 500Mcg/Ml | 500 mcg/ml | Injectable; Injection | Omega Laboratories Ltd | Prescription | DIN: 02248641 | 2004 |
| Octreotide Acetate Omega 50Mcg/Ml | 50 mcg/ml | Injectable; Injection | Omega Laboratories Ltd | Prescription | DIN: 02248639 | 2004 |
| Octreotide Injection | 50 mcg/ml | Injectable; Injection | Teva Canada Limited | Prescription | DIN: 02299429 | 2014 |
| Octreotide Injection | 100 mcg/ml | Injectable; Injection | Teva Canada Limited | Prescription | DIN: 02299437 | 2008 |
| Octreotide Injection | 200 mcg/ml | Injectable; Injection | Teva Canada Limited | Prescription | DIN: 02299445 | 2008 |
| Octreotide Injection | 500 mcg/ml | Injectable; Injection | Teva Canada Limited | Prescription | DIN: 02299453 | 2008 |
| Sandostatin 100Mcg/Ml | 100 mcg/ml | Injectable; Injection | Novartis Pharmaceuticals Canada Inc | Prescription | DIN: 00839205 | 1989 |
| Sandostatin 200Mcg/Ml | 200 mcg/ml | Injectable; Injection | Novartis Pharmaceuticals Canada Inc | Prescription | DIN: 02049392 | 1996 |
| Sandostatin 500Mcg/Ml | 500 mcg/ml | Injectable; Injection | Novartis Pharmaceuticals Canada Inc | Prescription | DIN: 00839213 | 1989 |
| Sandostatin 50Mcg/Ml | 50 mcg/ml | Injectable; Injection | Novartis Pharmaceuticals Canada Inc | Prescription | DIN: 00839191 | 1989 |
| Sandostatin Lar 10Mg | 10 mcg/vial | Powder; Intramuscular | Novartis Pharmaceuticals Canada Inc | Prescription | DIN: 02239323 | 1999 |
| Sandostatin Lar 20Mg | 20 mcg/vial | Powder; Intramuscular | Novartis Pharmaceuticals Canada Inc | Prescription | DIN: 02239324 | 1999 |
| Sandostatin Lar 30Mg | 30 mcg/vial | Powder; Intramuscular | Novartis Pharmaceuticals Canada Inc | Prescription | DIN: 02239325 | 1999 |
| Sandostatin | 1 ml: 0.1 mg | Injectable; Injection | Novartis Pharma Schweiz Ag | Prescription | NMPA: H20150364 | 1996 |
| ClinicalTrials.gov Identifier | Title | Condition or disease | Phase | Purpose |
|---|---|---|---|---|
| NCT03289741 | A Pilot Study To Evaluate Patient Experience With the Somatostatin Analogs Octreotide Long Acting Release and Lanreotide During the Treatment of Advanced, Nonfunctional, Well Differentiated Neuroendocrine Tumors | Neuroendocrine Tumors | Phase 4 | Treatment |
| NCT02409849 | Randomized Phase II Study of Octreotide LAR as Maintenance Treatment After First-line Chemotherapy for Patients With Unresectable or Metastatic Gastro-entero-pancreatic or Esophageal Neuroendocrine Carcinomas | Gastro-entero-pancreatic Carcinoma; Esophageal Neuroendocrine Carcinoma | Phase 2 | Treatment |
| NCT05459844 | A Study Comparing Treatment With Lutetium[177Lu] Oxodotreotide Injection to Octreotide LAR in Patients With Inoperable, Progressive, , Well Differentiated, Somatostatin Receptor Positive Gastroenteropancreatic Neuroendocrine Tumours | Neuroendocrine Tumors | Phase 3 | Treatment |
| NCT01578239 | A Multicentre, Stratified, Open, Randomized, Comparator-controlled, Parallel-group Phase III Study Comparing Treatment With 177Lu-DOTA0-Tyr3-Octreotate to Octreotide LAR in Patients With Inoperable, Progressive, Somatostatin Receptor Positive Midgut Carcinoid Tumours | Carcinoid Tumor of the Small Bowel; Neuroendocrine Tumour | Phase 3 | Treatment |
| NCT00427349 | A Phase II Clinical and Biologic Study of AMG 706 and Octreotide in Patients With Low-Grade Neuroendocrine Tumors | Gastrointestinal Carcinoid Tumor; Islet Cell Tumor; Neoplastic Syndrome | Phase 2 | Treatment |
More clinical information is obtained from ClinicalTrials.gov.