Lanreotide
DRACPC ID DRACPC0005
Active Ingredients Lanreotide
Description A synthetic cyclic octapeptide analogue of somatostatin. Lanreotide binds to somatostatin receptors (SSTR), specifically SSTR-2 and also to SSTR-5 with a lesser affinity. However, compare with octreotide, this agent is less potent in inhibiting the release of growth hormone from the pituitary gland. Furthermore, lanreotide has an acute effect on decreasing circulating total and free insulin-like growth factor 1 (IGF-I). This agent is usually given as a prolonged-release microparticle or Autogel formulation for the treatment of acromegaly and to relieve the symptoms of neuroendocrine tumors.
Synonyms Abarelix; Plenaxis; Angiopeptin; BIM-23014; DC 13116; Dermopeptin; Ipstyl; L-Threoninamide, 3-(2-naphthalenyl)-D-alanyl-L-cysteinyl-L-tyrosyl-D-tryptophyl-L-lysyl-L-valyl-L-cysteinyl-, cyclic (2-7)-disulfide; Nal-cyclo(cys-tyr-trp-lys-val-cys)-thr-NH2; Somatulina; Somatuline; Lanreotide
Type Small Molecule
Disease Gastroenteropancreatic neuroendocrine tumors, Acromegaly
Classification
Somatostatin and analogues Peptide and derivative Hormone and analogue
Structure Information
Molecular Formula C54H69N11O10S2
Molecular Weight 1096.333
Active Sequence XCYWKVCX
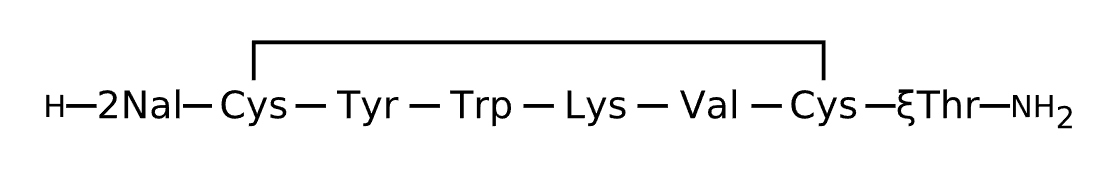
Sequence Length 8
Modification X(1)=2Nal, X(8)=xiThr, N-terminal NH2
IUPAC Name 3-(2-naphthyl)-D-alanyl-L-cysteinyl-L-tyrosyl-D-tryptophyl-L-lysyl-L-valyl-L-cysteinyl-L-threoninamide (2->7)-disulfide
InChI InChI=1S/C54H69N11O10S2/c1-29(2)45-54(75)63-44(53(74)65-46(30(3)66)47(57)68)28-77-76-27-43(62-48(69)38(56)23-32-15-18-33-10-4-5-11-34(33)22-32)52(73)60-41(24-31-16-19-36(67)20-17-31)50(71)61-42(25-35-26-58-39-13-7-6-12-37(35)39)51(72)59-40(49(70)64-45)14-8-9-21-55/h4-7,10-13,15-20,22,26,29-30,38,40-46,58,66-67H,8-9,14,21,23-25,27-28,55-56H2,1-3H3,(H2,57,68)(H,59,72)(H,60,73)(H,61,71)(H,62,69)(H,63,75)(H,64,70)(H,65,74)
InChI_Key PUDHBTGHUJUUFI-UHFFFAOYSA-N
SMILES CC(C1NC(C(NC(C(NC(C(NC(C(CSSCC(NC1=O)C(NC(C(O)C)C(N)=O)=O)NC(C(CC2=CC=C3C=CC=CC3=C2)N)=O)=O)CC4=CC=C(C=C4)O)=O)CC5=CNC6=CC=CC=C56)=O)CCCCN)=O)C
External Codes
PubChem CID 6918011
DrugBank Accession Number DB06791
NCI Thesaurus Code C1523
UNII 0G3DE8943Y GSRS
CAS 108736-35-2
Drug approval
Drug indication
Lanreotide is indicated for the long-term treatment of patients with acromegaly who have had an inadequate response to, or cannot be treated with, surgery and/or radiotherapy. It is also indicated in the treatment of adult patients with unresectable, well- or moderately-differentiated, locally advanced or metastatic gastroenteropancreatic neuroendocrine tumors (GEP-NETs) to improve progression-free survival.
Lanreotide is additionally indicated for the treatment of adults with carcinoid syndrome - when used, it reduces the frequency of short-acting somatostatin analog rescue therapy.
| Drug Name | Strength | Dosage Form/Route | Company | Marketing Status | Drug ID | Approval year |
|---|---|---|---|---|---|---|
| Somatuline Depot | eq 60mg base/0.2ml; eq 90mg base/0.3ml; eq 120mg base/0.5ml | Solution; Subcutaneous | Ipsen Pharma | Prescription | NDA: 022074 | 2007 |
| Somatuline Autogel | 60 mg/syr | Solution; Subcutaneous | Ipsen Biopharm Limited | Prescription | DIN: 02283395 | 2007 |
| Somatuline Autogel | 90 mg/syr | Solution; Subcutaneous | Ipsen Biopharm Limited | Prescription | DIN: 02283409 | 2007 |
| Somatuline Autogel | 120 mg/syr | Solution; Subcutaneous | Ipsen Biopharm Limited | Prescription | DIN: 02283417 | 2007 |
| ClinicalTrials.gov Identifier | Title | Condition or disease | Phase | Purpose |
|---|---|---|---|---|
| NCT03946527 | Exploratory Phase II Study of LAnreotide in Metastatic Pheochromocytoma/PARAganglioma (LAMPARA) | Paraganglioma; Pheochromocytoma | Phase 2 | Treatment |
| NCT04427787 | A Phase II Trial Aiming to Assess the Safety and Activity of the Combination of Cabozantinib Plus Lanreotide in Gastroenteropancreatic (GEP) and Thoracic Neuroendocrine Tumor (NET): The LOLA Trial | Metastatic Well Differentiated Neuroendocrine Neoplasm; Neuroendocrine Tumors | Phase 2 | Treatment |
| NCT02651987 | Efficacy and Safety of Lanreotide Autogel® 120 mg Administered Every 14 Days in Well Differentiated, Metastatic or Locally Advanced, Unresectable Pancreatic or Midgut Neuroendocrine Tumours Having Progressed Radiologically While Previously Treated With Lanreotide Autogel® 120 mg Administered Every 28 Days | Pancreatic Tumours; Midgut Neuroendocrine Tumours | Phase 2 | Treatment |
| NCT03043664 | Phase Ib/II Study of Pembrolizumab With Lanreotide Depot for Gastroenteropancreatic Neuroendocrine Tumors | Gastroenteropancreatic Neuroendocrine Tumors | Phase 1/2 | Treatment |
| NCT00842348 | Open Label Extension Study of Lanreotide Autogel 120 mg in Patients With Non-functioning Entero-pancreatic Endocrine Tumour | Non Functioning Entero-pancreatic Endocrine Tumour | Phase 3 | Treatment |
More clinical information is obtained from ClinicalTrials.gov.