Abarelix
DRACPC ID DRACPC0006
Active Ingredients Abarelix
Description A synthetic decapeptide and antagonist of naturally occurring gonadotropin-releasing hormone (GnRH). Abarelix directly and competitively binds to and blocks the gonadotropin releasing hormone receptor in the anterior pituitary gland, thereby inhibiting the secretion and release of luteinizing hormone (LH) and follicle stimulating hormone (FSH). In males, the inhibition of LH secretion prevents the release of testosterone. As a result, this may relieve symptoms associated with prostate hypertrophy or prostate cancer, since testosterone is required to sustain prostate growth.
Synonyms Plenaxis; PPI-149; R-3827; Abarelix
Type Small Molecule
Disease Prostate cancer
Classification
GnRH antagonists Peptide and derivative
Structure Information
Molecular Formula C72H95ClN14O14
Molecular Weight 1416.1
Active Sequence xxxSXnLXPa
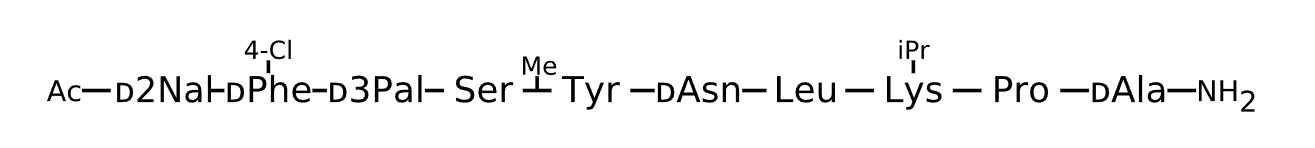
Sequence Length 10
Modification x(1)=D-2Nal, x(2)=D-Phe(4-Cl), x(3)=D-3Pal, X(5)=N(Me)Tyr, X(8)=Lys(iPr), C-terminal Ac, N-terminal NH2
IUPAC Name (2R)-2-[[(2S)-2-[[(2S)-2-[[(2R)-2-[[(2R)-2-[[(2R)-2-acetamido-3-naphthalen-2-ylpropanoyl]amino]-3-(4-chlorophenyl)propanoyl]amino]-3-pyridin-3-ylpropanoyl]amino]-3-hydroxypropanoyl]-methylamino]-3-(4-hydroxyphenyl)propanoyl]amino]-N-[(2S)-1-[[(2S)-1-[(2S)-2-[[(2R)-1-amino-1-oxopropan-2-yl]carbamoyl]pyrrolidin-1-yl]-1-oxo-6-(propan-2-ylamino)hexan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]butanediamide
InChI InChI=1S/C72H95ClN14O14/c1-41(2)32-54(64(93)80-53(17-10-11-30-77-42(3)4)72(101)87-31-13-18-60(87)69(98)78-43(5)63(75)92)81-68(97)58(38-62(74)91)84-70(99)61(37-46-22-27-52(90)28-23-46)86(7)71(100)59(40-88)85-67(96)57(36-48-14-12-29-76-39-48)83-66(95)56(34-45-20-25-51(73)26-21-45)82-65(94)55(79-44(6)89)35-47-19-24-49-15-8-9-16-50(49)33-47/h8-9,12,14-16,19-29,33,39,41-43,53-61,77,88,90H,10-11,13,17-18,30-32,34-38,40H2,1-7H3,(H2,74,91)(H2,75,92)(H,78,98)(H,79,89)(H,80,93)(H,81,97)(H,82,94)(H,83,95)(H,84,99)(H,85,96)/t43-,53+,54+,55-,56-,57-,58-,59+,60+,61+/m1/s1
InChI_Key AIWRTTMUVOZGPW-HSPKUQOVSA-N
SMILES CC(N[C@@H](C(N[C@@H](C(N[C@@H](C(N[C@H](C(N([C@H](C(N[C@@H](C(N[C@H](C(N[C@H](C(N1CCC[C@H]1C(N[C@@H](C(N)=O)C)=O)=O)CCCCNC(C)C)=O)CC(C)C)=O)CC(N)=O)=O)CC2=CC=C(C=C2)O)C)=O)CO)=O)CC3=CC=CN=C3)=O)CC4=CC=C(C=C4)Cl)=O)CC5=CC=C6C=CC=CC6=C5)=O
External Codes
PubChem CID 16131215
DrugBank Accession Number DB00106
NCI Thesaurus Code C2015
UNII W486SJ5824 GSRS
CAS 183552-38-7
Drug approval
Drug indication
For palliative treatment of advanced prostate cancer.
| Drug Name | Strength | Dosage Form/Route | Company | Marketing Status | Drug ID | Approval year |
|---|---|---|---|---|---|---|
| Plenaxis | 100 mg/vial | Injectable; Intramuscular | Speciality European | Discontinued | NDA: 021320 | 2003 |
| ClinicalTrials.gov Identifier | Title | Condition or disease | Phase | Purpose |
|---|---|---|---|---|
| NCT00841113 | Phase III Study of the Comparison of Abarelix Versus Goserelin Plus Bicalutamide in Patients With Advanced or Metastatic Prostate Cancer. A One Year Randomised, Open Label, Multi-Centre Phase III Trial. | Prostate Cancer | Phase 3 | Treatment |
| NCT00100243 | Phase 2 Study of Abarelix in Androgen-Independent Prostate Cancer Progressing After Agonist Therapy | Prostate Cancer | Phase 2 | Treatment |
| NCT00103623 | Incidence of Immediate Onset Systemic Allergic Reactions in Patients Treated With Plenaxis® | Prostate Cancer | Phase 4 | Treatment |
More clinical information is obtained from ClinicalTrials.gov.