Degarelix
DRACPC ID DRACPC0007
Active Ingredients Degarelix
Description A long-acting, synthetic peptide with gonadotrophin-releasing hormone (GnRH) antagonistic properties. Degarelix targets and blocks GnRH receptors located on the surfaces of gonadotroph cells in the anterior pituitary, thereby reducing secretion of luteinizing hormone (LH) by pituitary gonadotroph cells and so decreasing testosterone production by interstitial (Leydig) cells in the testes. Chemically, Degarelix is a synthetic linear decapeptide amide with seven unnatural amino acids, five of which are D-amino acids.
Synonyms FE200486; Firmagon; N-acetyl-3-(naphtalen-2-yl)-D-alanyl-4-chloro-D-phenylalanyl-3-(pyridin-3-yl)-D-alanyl-L-seryl-4-((((4S)-2,6-dioxohexahydropyrimidin-4-yl)carbonyl)amino)-L-phenylalanyl-4-(carbamoylamino)-D-phenylalanyl-L-leucyl-N6-(1-methylethyl)-L-lysyl-L-prolyl-D-alaninamide; Degarelix
Type Small Molecule
Disease Prostate cancer
Classification
GnRH antagonist Peptide and derivative
Structure Information
Molecular Formula C82H103ClN18O16
Molecular Weight 1632.286
Active Sequence xxxSXxLXPa
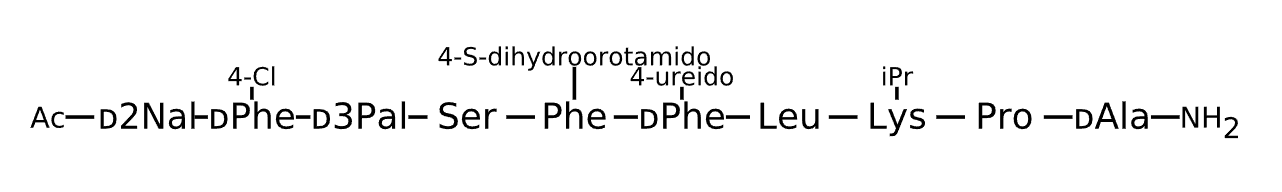
Sequence Length 10
Modification x(1)=D-2Nal, x(2)=D-Phe(4-Cl), x(3)=D-3Pal, X(5)=Phe(4-S-dihydroorotamido), x(6)=D-Phe(4-ureido), X(8)=Lys(iPr), C-terminal Ac, N-terminal NH2
IUPAC Name (4S)-N-{4-[(2S)-2-{[(1R)-2-[4-(carbamoylamino)phenyl]-1-{[(1S)-1-{[(2S)-1-[(2S)-2-{[(1R)-1-carbamoylethyl]carbamoyl}pyrrolidin-1-yl]-1-oxo-6-[(propan-2-yl)amino]hexan-2-yl]carbamoyl}-3-methylbutyl]carbamoyl}ethyl]carbamoyl}-2-[(2S)-2-[(2R)-2-[(2R)-3-(4-chlorophenyl)-2-[(2R)-2-acetamido-3-(naphthalen-2-yl)propanamido]propanamido]-3-(pyridin-3-yl)propanamido]-3-hydroxypropanamido]ethyl]phenyl}-2,6-dioxo-1,3-diazinane-4-carboxamide
InChI InChI=1S/C82H103ClN18O16/c1-45(2)35-60(72(107)92-59(16-9-10-33-87-46(3)4)80(115)101-34-12-17-68(101)79(114)88-47(5)70(84)105)93-74(109)63(38-51-23-30-58(31-24-51)91-81(85)116)95-76(111)64(39-50-21-28-57(29-22-50)90-71(106)66-42-69(104)100-82(117)99-66)97-78(113)67(44-102)98-77(112)65(41-53-13-11-32-86-43-53)96-75(110)62(37-49-19-26-56(83)27-20-49)94-73(108)61(89-48(6)103)40-52-18-25-54-14-7-8-15-55(54)36-52/h7-8,11,13-15,18-32,36,43,45-47,59-68,87,102H,9-10,12,16-17,33-35,37-42,44H2,1-6H3,(H2,84,105)(H,88,114)(H,89,103)(H,90,106)(H,92,107)(H,93,109)(H,94,108)(H,95,111)(H,96,110)(H,97,113)(H,98,112)(H3,85,91,116)(H2,99,100,104,117)/t47?,59-,60-,61+,62+,63+,64-,65?,66?,67-,68-/m0/s1
InChI_Key MEUCPCLKGZSHTA-CTZYXKPMSA-N
SMILES CC(NCCCC[C@H](NC([C@@H](NC([C@H](NC([C@@H](NC([C@@H](NC(C(NC([C@H](NC([C@H](NC(C)=O)CC1=CC=C2C=CC=CC2=C1)=O)CC3=CC=C(Cl)C=C3)=O)CC4=CN=CC=C4)=O)CO)=O)CC5=CC=C(NC(C(NC(N6)=O)CC6=O)=O)C=C5)=O)CC7=CC=C(NC(N)=O)C=C7)=O)CC(C)C)=O)C(N8CCC[C@@]8([H])C(NC(C)C(N)=O)=O)=O)C
External Codes
PubChem CID 16136245
DrugBank Accession Number DB06699
NCI Thesaurus Code C48385
UNII SX0XJI3A11 GSRS
CAS 214766-78-6
Drug approval
Drug indication
Degaralix is used for the treat of advanced prostate cancer and is also being studied in the treatment of benign prostatic hyperplasia.
| Drug Name | Strength | Dosage Form/Route | Company | Marketing Status | Drug ID | Approval year |
|---|---|---|---|---|---|---|
| Firmagon | eq 80mg base/vial; eq 120mg base/vial | Powder; Subcutaneous | Ferring | Prescription | NDA: 022201 | 2008 |
| Firmagon | eq 120mg base/vial | Powder; Subcutaneous | Ferring | Prescription | NDA: 022202 | 2008 |
| Firmagon | 80 mg/vial | Powder; Subcutaneous | Ferring Inc | Prescription | DIN: 02337029 | 2009 |
| Firmagon | 120 mg/vial | Powder; Subcutaneous | Ferring Inc | Prescription | DIN: 02337037 | 2009 |
| Firmagon | 80 mg/vial; 120 mg/vial | Powder; Subcutaneous | Ferring Pharmaceuticals | Prescription | EMEA/H/C/000986 | 2009 |
| ClinicalTrials.gov Identifier | Title | Condition or disease | Phase | Purpose |
|---|---|---|---|---|
| NCT03069937 | A Phase II Study of Docetaxel Before Medical Castration With Degarelix in Patients With Newly Diagnosed Metastatic Prostatic Adenocarcinoma. | Metastatic Prostatic Adenocarcinoma | Phase 2 | Treatment |
| NCT02475057 | A Pilot Study on Endothelial Function and Cardiovascular Biomarkers in Prostate Cancer (PCa) Patients, With Pre-existing Cardiovascular Disease, Treated With Degarelix vs. Luteinizing Hormone-Releasing Hormone (LHRH) Agonists | Prostatic Neoplasms; Cardiovascular Diseases | Phase 4 | Treatment |
| NCT01512472 | Randomized, Multicentre Efficacy and Safety Study Comparing 10 Mons vs 4 Mons Degarelix Therapy in Prolonging the Off Treatment Interval in Men With Localized Prostate Cancer Receiving Intermittent ADT for Biochemical Recurrence Following Radical Local Therapy | Prostate Cancer Recurrent | Phase 4 | Treatment |
| NCT02015871 | An Extension Long-Term Safety and Tolerability Trial of Degarelix, Following a 1-year Open-Label, Multi-Centre, Randomised, Parallel-group Trial in Which the Efficacy and Safety of Degarelix One-month Dosing Regimen Was Compared With Goserelin in Chinese Patients With Prostate Cancer Requiring Androgen Ablation Therapy | Prostate Cancer | Phase 3 | Treatment |
| NCT01994239 | A Multicenter Randomised Phase II Study Comparing the Efficiency of a HT Concomitant With RT vs RT Alone in the Salvage of Patients With a Detectable PSA After Prostatectomy | Adenocarcinoma of Prostate | Phase 2 | Treatment |
More clinical information is obtained from ClinicalTrials.gov.