Somatostatin
DRACPC ID DRACPC0008
Active Ingredients Somatostatin
Description Somatostatin, also known as growth hormone-inhibiting hormone, is a naturally-occurring peptide hormone of 14 or 28 amino acid residues that regulates the endocrine system. It is secreted by the D cells of the islets to inhibit the release of insulin and glucagon, and is also generated in the hypothalamus, where it inhibits the release of growth hormone and thyroid-stimulating hormones from the anterior pituitary. Somatostatin is initially secreted as a 116 amino acid precursor, preprosomatostatin, which undergoes endoproteolytic cleavage to prosomastatin. Prosomastatin is further process into two active forms, somatostatin-14 (SST-14) and somatostatin-28 (SST-28), an extended SST-14 sequence to the N-terminus. The actions of somatostatin are mediated via signalling pathways of G protein-coupled somatostatin receptors. Antineoplastic effects and potential uses of somatostatin on various tumours, including pituitary adenomas, GEP-NETs, paragangliomas, carcinoids, breast cancers, malignant lymphoma and small-cell lung cancers, have been extensively investigated. Somatostatin has been used in the clinical setting for the diagnosis of acromegaly and gastrointestinal tract tumours. Its analogues have been developed to achieve more favourable kinetics for efficiency use in the management of acute conditions, such as esophageal varices. Octreotide is a long-acting analogue of somatostatin that inhibits the release of a number of hormones, and is clinically used to relieve symptoms of uncommon gastroenteropancreatic endocrine tumours, as well as treat acromegaly.
Synonyms AY 24910; GH-RIH; Growth Hormone Inhibiting Hormone; Panhibin; Recombinant Somatostatin; Somatostatin
Type Small Molecule
Disease Various tumours, Oesophageal varices haemorrhage, Gastritis haemorrhagic, Acromegaly
Classification
Somatostatin and analogues Peptide and derivative Hormone and analogue Cyclic
Structure Information
Molecular Formula C76H104N18O19S2
Molecular Weight 1637.895
Active Sequence AGCKNFFWKTFTSC
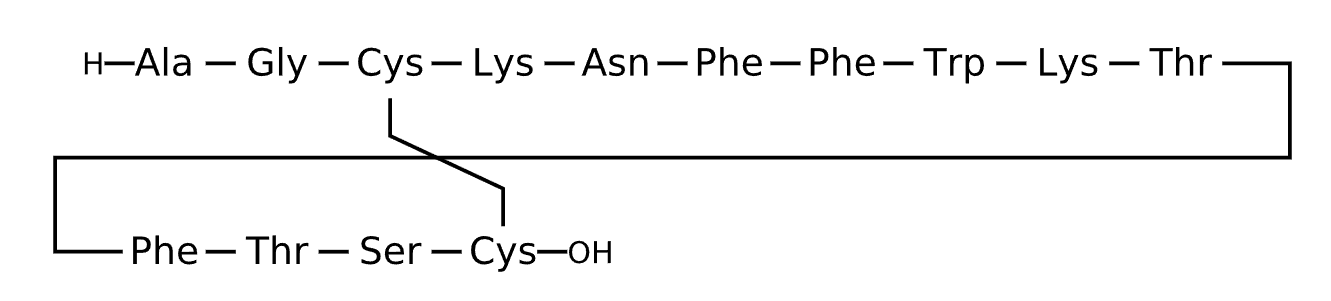
Sequence Length 14
Modification None
IUPAC Name (4R,7S,10S,13S,16S,19S,22S,25S,28S,31S,34S,37R)-19,34-bis(4-aminobutyl)-37-{2-[(2S)-2-aminopropanamido]acetamido}-13,25,28-tribenzyl-31-(carbamoylmethyl)-10,16-bis[(1R)-1-hydroxyethyl]-7-(hydroxymethyl)-22-[(1H-indol-3-yl)methyl]-6,9,12,15,18,21,24,27,30,33,36-undecaoxo-1,2-dithia-5,8,11,14,17,20,23,26,29,32,35-undecaazacyclooctatriacontane-4-carboxylic acid
InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1
InChI_Key NHXLMOGPVYXJNR-ATOGVRKGSA-N
SMILES C[C@@H](C(NCC(N[C@H]1CSSC[C@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](NC1=O)CCCCN)=O)CC(N)=O)=O)CC2=CC=CC=C2)=O)CC3=CC=CC=C3)=O)CC4=CNC5=CC=CC=C45)=O)CCCCN)=O)[C@H](O)C)=O)CC6=CC=CC=C6)=O)[C@H](O)C)=O)CO)=O)C(O)=O)=O)=O)N
External Codes
PubChem CID 16129706
DrugBank Accession Number DB09099
NCI Thesaurus Code C836
UNII 6E20216Q0L GSRS
CAS 38916-34-6
Drug approval
Drug indication
For the symptomatic treatment of acute bleeding from esophageal varices, gastrointestinal ulcers, and gastritis; prevent pancreatic complications after surgery; and restrict secretions of the upper intestine, pancreas, and biliary tract. Somatostatin have been extensively investigated for use/treatment in various tumours, including pituitary adenomas, GEP-NETs, paragangliomas, carcinoids, breast cancers, malignant lymphoma and small-cell lung cancers.
| Drug Name | Strength | Dosage Form/Route | Company | Marketing Status | Drug ID | Approval year |
|---|---|---|---|---|---|---|
| Stilamin - Pws Iv 3Mg/Amp | 3 mg/amp | Solution; Intravenous | Emd Serono A Division Of Emd Inc Canada | Discontinued | DIN: 02125277 | 1997 |
| Stilamin - Pws Liq Iv | 250 mcg/kit | Solution; Intravenous | Emd Serono A Division Of Emd Inc Canada | Discontinued | DIN: 02125269 | 1997 |
| ClinicalTrials.gov Identifier | Title | Condition or disease | Phase | Purpose |
|---|---|---|---|---|
| NCT01053390 | A Phase III Study of New Chemotherapy Regimen in the Treatment of Advanced Gallbladder Carcinoma | Gallbladder Neoplasms | Phase 3 | Treatment |
| NCT02631616 | Treatment With Sandostatin in Patients With Castrate Resistance Prostate Cancer Showing Uptake of 68Ga-DOTATET | Prostatic Neoplasms | Phase 1 | Treatment |
| NCT01914692 | Application of Somatostatin for Advanced Gastric Cancer After D2 Lymph Node Dissection -a Prospective Randomized Controlled Study | Gastric Cancer After D2 Lymph Node Dissection | Phase 4 | Treatment |
| NCT01053390 | A Phase III Study of New Chemotherapy Regimen in the Treatment of Advanced Gallbladder Carcinoma | Gallbladder Neoplasms | Phase 3 | Treatment |
| NCT03000946 | Prevention of Postoperative Pancreatic Fistula by SOMATOSTATIN Compared to OCTREOTIDE: Prospective, Randomized, Controlled Study | Pancreatic Surgery | Phase 3 | Prevention |
More clinical information is obtained from ClinicalTrials.gov.