Buserelin
DRACPC ID DRACPC0009
Active Ingredients Buserelin
Description A synthetic analog of gonadotropin-releasing hormone (GnRH). Buserelin binds to and activates pituitary gonadotropin releasing hormone (GnRH) receptors. Prolonged administration of buserelin results in sustained inhibition of gonadotropin production, suppression of testicular and ovarian steroidogenesis, and reduced levels of circulating gonadotropin and gonadal steroids. Buserelin is more potent that GnRH.
Synonyms BSRL; Etilamide; HOE 766; ICI 123215; S74-6766; 6-[O-(1,1-Dimethylethyl)-D-serine]-9-(N-ethyl-L-prolinamide)-10-deglycinamide Luteinizing Hormone-Releasing Factor (Pig); Buserelin
Type Small Molecule
Disease Breast cancer, Contraception, female, Endometriosis, Infertility, in vitro fertilization, Polycystic ovary syndrome, Prostate cancer, Uterine leiomyoma
Classification
Peptide and derivative Hormone and analogue
Structure Information
Molecular Formula C60H86N16O13
Molecular Weight 1239.447
Active Sequence XHWSYxLRP
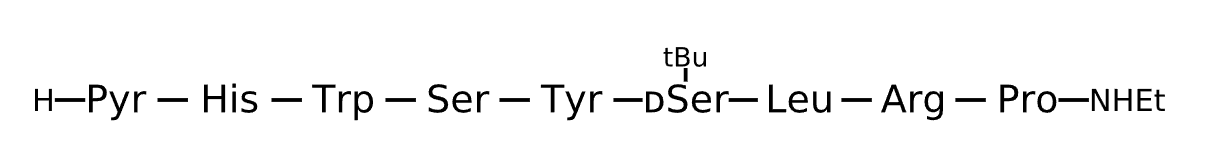
Sequence Length 9
Modification X=Pyr, x=Ser(tBu), N-terminal NHEt
IUPAC Name (2S)-N-[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2R)-1-[[(2S)-1-[[(2S)-5-(diaminomethylideneamino)-1-[(2S)-2-(ethylcarbamoyl)pyrrolidin-1-yl]-1-oxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-[(2-methylpropan-2-yl)oxy]-1-oxopropan-2-yl]amino]-3-(4-hydroxyphenyl)-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-3-(1H-imidazol-5-yl)-1-oxopropan-2-yl]-5-oxopyrrolidine-2-carboxamide
InChI InChI=1S/C60H86N16O13/c1-7-64-57(87)48-15-11-23-76(48)58(88)41(14-10-22-65-59(61)62)69-51(81)42(24-33(2)3)70-56(86)47(31-89-60(4,5)6)75-52(82)43(25-34-16-18-37(78)19-17-34)71-55(85)46(30-77)74-53(83)44(26-35-28-66-39-13-9-8-12-38(35)39)72-54(84)45(27-36-29-63-32-67-36)73-50(80)40-20-21-49(79)68-40/h8-9,12-13,16-19,28-29,32-33,40-48,66,77-78H,7,10-11,14-15,20-27,30-31H2,1-6H3,(H,63,67)(H,64,87)(H,68,79)(H,69,81)(H,70,86)(H,71,85)(H,72,84)(H,73,80)(H,74,83)(H,75,82)(H4,61,62,65)/t40-,41-,42-,43-,44-,45-,46-,47+,48-/m0/s1
InChI_Key CUWODFFVMXJOKD-UVLQAERKSA-N
SMILES O=C(N1)CC[C@H]1C(N[C@H](C(N[C@H](C(N[C@@H](CO)C(N[C@H](C(N[C@H](COC(C)(C)C)C(N[C@H](C(N[C@@H](CCCNC(N)=N)C(N2[C@@H](CCC2)C(NCC)=O)=O)=O)CC(C)C)=O)=O)CC3=CC=C(O)C=C3)=O)=O)CC4=CNC5=C4C=CC=C5)=O)CC6=CNC=N6)=O
External Codes
PubChem CID 50225
DrugBank Accession Number DB06719
NCI Thesaurus Code C320
UNII PXW8U3YXDV GSRS
CAS 57982-77-1
Drug approval
Drug indication
Buserelin may be used in the treatment of hormone-responsive cancers such as prostate cancer or breast cancer, estrogen-dependent conditions (such as endometriosis or uterine fibroids), and in assisted reproduction.
| Drug Name | Strength | Dosage Form/Route | Company | Marketing Status | Drug ID | Approval year |
|---|---|---|---|---|---|---|
| Suprefact | 1 mg/ml | Solution; Nasal | Sanofi-Aventis Canada Inc | Prescription | DIN: 02225158 | 1998 |
| Suprefact | 1 mg/ml | Solution; Subcutaneous | Sanofi-Aventis Canada Inc | Prescription | DIN: 02225166 | 1996 |
| Suprefact Depot 2 Months | 6.3 mg/imp | Implant; Subcutaneous | Sanofi-Aventis Canada Inc | Prescription | DIN: 02228955 | 1997 |
| Suprefact Depot 3 Months | 9.45 mg/imp | Implant; Subcutaneous | Sanofi-Aventis Canada Inc | Prescription | DIN: 02240749 | 2000 |
| Suprefact Inj 1Mg/Ml | 1 mg/ml | Solution; Subcutaneous | Sanofi-Aventis Canada Inc | Discontinued | DIN: 00680028 | 1988 |
| Suprefact Intranasal Solution 1Mg/Ml | 1 mg/ml | Spray, Metered; Nasal | Hoechst Canada Inc. | Discontinued | DIN: 00680036 | 1988 |
| Suprefact Liq 1Mg/Ml | 1 mg/ml | Spray, Metered; Nasal | Hoechst Roussel Canada Inc. | Discontinued | DIN: 01989669 | 1993 |
| ClinicalTrials.gov Identifier | Title | Condition or disease | Phase | Purpose |
|---|---|---|---|---|
| NCT00054106 | A Phase I Study Of Combination Neoadjuvant Hormone Therapy And Weekly OGX-011 (Clusterin Antisense Oligonucleotide) Prior To Radical Prostatectomy In Patients With Localized Prostate Cancer | Prostate Cancer | Phase 1 | Treatment |
| NCT00002633 | Phase III Randomized Trial Comparing Total Androgen Blockade Versus Total Androgen Blockade Plus Pelvic Irradiation in Clinical Stage T3-4, N0, M0 Adenocarcinoma of the Prostate | Prostate Cancer | Phase 3 | Treatment |
| NCT01546987 | Phase III Trial of Dose Escalated Radiation Therapy and Standard Androgen Deprivation Therapy (ADT) With a GNRH Agonist vs. Dose Escalated Radiation Therapy and Enhanced ADT With a GNRH Agonist and TAK-700 For Men With High Risk Prostate Cancer | Prostate Cancer | Phase 3 | Treatment |
| NCT05050084 | Parallel Phase III Randomized Trials of Genomic-Risk Stratified Unfavorable Intermediate Risk Prostate Cancer: De-Intensification and Intensification Clinical Trial Evaluation (GUIDANCE) | Prostate Adenocarcinoma | Phase 3 | Treatment |
| NCT00627406 | A Prospective Randomised Study to Evaluate the Effect of Triggering Ovulation With GnRHa (Buserelin) and Low Dose hCG (Pregnyl) as Compared to the Use of Conventional Doses of hCG (Pregnyl) | OHSS (Ovarian Hyperstimulation) | Phase 4 | Treatment |
More clinical information is obtained from ClinicalTrials.gov.