Sovateltide
DRACPC ID DRACPC0015
Active Ingredients Sovateltide
Description A highly selective peptide agonist of the endothelin-B receptor. Sovateltide binds to endothelin-B receptors on endothelial cells in tumor blood vessels, which, unlike the angioarchitecture of normal blood vessels, are relatively devoid of smooth muscle. This agent may induce a transient, selective increase in blood flow to a tumor, which may result in an increase in the delivery of anticancer agents to the tumor and, so, an increase in anticancer agent efficacy.
Synonyms SPI-1620; IRL-1620; Endothelin B Receptor Agonist SPI-1620; Sovateltide; PMZ-1620
Type Small Molecule
Disease Cancer/Tumor
Classification
Endothelin-B receptor agonist Peptide and derivative
Structure Information
Molecular Formula C86H117N17O27
Molecular Weight 1820.9
Active Sequence DEEAVYFAHLDIIW
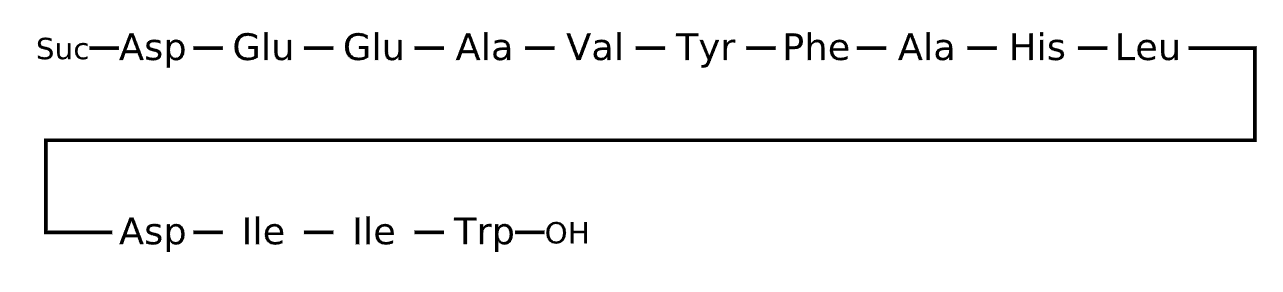
Sequence Length 14
Modification C-terminal Suc
IUPAC Name N-(3-carboxypropanoyl)-L-alpha-aspartyl-L-alpha-glutamyl-L-alpha-glutamyl-L-alanyl-L-valyl-L-tyrosyl-L-phenylalanyl-L-alanyl-L-histidyl-L-leucyl-L-alpha-aspartyl-L-isoleucyl-L-isoleucyl-L-tryptophan
InChI InChI=1S/C86H117N17O27/c1-11-44(7)71(84(127)100-63(86(129)130)35-50-39-88-54-21-17-16-20-53(50)54)103-85(128)72(45(8)12-2)102-82(125)62(38-69(114)115)98-78(121)57(32-42(3)4)96-80(123)60(36-51-40-87-41-89-51)95-73(116)46(9)91-77(120)58(33-48-18-14-13-15-19-48)97-79(122)59(34-49-22-24-52(104)25-23-49)99-83(126)70(43(5)6)101-74(117)47(10)90-75(118)55(26-29-65(106)107)93-76(119)56(27-30-66(108)109)94-81(124)61(37-68(112)113)92-64(105)28-31-67(110)111/h13-25,39-47,55-63,70-72,88,104H,11-12,26-38H2,1-10H3,(H,87,89)(H,90,118)(H,91,120)(H,92,105)(H,93,119)(H,94,124)(H,95,116)(H,96,123)(H,97,122)(H,98,121)(H,99,126)(H,100,127)(H,101,117)(H,102,125)(H,103,128)(H,106,107)(H,108,109)(H,110,111)(H,112,113)(H,114,115)(H,129,130)/t44-,45-,46-,47-,55-,56-,57-,58-,59-,60-,61-,62-,63-,70-,71-,72-/m0/s1
InChI_Key DXPHNGAMYPPTBJ-TZMIJSMNSA-N
SMILES CC[C@@H]([C@@H](C(N[C@@H]([C@H](CC)C)C(N[C@H](C(O)=O)CC1=CNC2=CC=CC=C12)=O)=O)NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@H](C(C)C)NC([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](NC(CCC(O)=O)=O)CC(O)=O)=O)CCC(O)=O)=O)CCC(O)=O)=O)C)=O)=O)CC3=CC=C(C=C3)O)=O)CC4=CC=CC=C4)=O)C)=O)CC5=CNC=N5)=O)CC(C)C)=O)CC(O)=O)=O)C
External Codes
PubChem CID 16133819
DrugBank Accession Number DB06138
NCI Thesaurus Code C74027
UNII 11X778QIZS GSRS
CAS 142569-99-1
Drug approval
Drug indication
Investigated for use/treatment in cancer/tumors (unspecified).
The drug is not approved.
| ClinicalTrials.gov Identifier | Title | Condition or disease | Phase | Purpose |
|---|---|---|---|---|
| NCT01741155 | A 2-Part Phase 2 Study of SPI-1620 in Combination With Docetaxel Versus Docetaxel Alone for Patients With Non Small-cell Lung Cancer After Failure of First-line Platinum-based Chemotherapy | Non Small Cell Lung Cancer (NSCLC) | Phase 2 | Treatment |
| NCT01773785 | Phase II Study of SPI-1620 in Combination With Docetaxel as a Second-Line Treatment for Patients With Advanced Biliary Cancer | Biliary Cancer | Phase 2 | Treatment |
| NCT00613691 | A Phase I, Open-label, Ascending Dose Study of the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of the Endothelin B Agonist, SPI-1620, in Patients With Recurrent or Progressive Carcinoma | Carcinoma | Phase 1 | Treatment |
More clinical information is obtained from ClinicalTrials.gov.