Histrelin
DRACPC ID DRACPC0018
Active Ingredients Histrelin
Description A long-acting, synthetic nonapeptide analog of gonadotropin-releasing hormone (GnRH) with potential anti-tumor activity. Upon administration, histrelin binds to and activates GnRH receptors; prolonged administration results in pituitary GnRH receptor desensitization and inhibition of follicle stimulating hormone (FSH) and luteinizing hormone (LH) secretion, leading to a significant decline in testosterone production in males and may inhibit androgen receptor-positive tumor progression; in females, prolonged administration results in decreased estradiol production.
Synonyms ORF 17070, RWJ 17070; Histrelin
Type Small Molecule
Disease Central precocious puberty(CPP), Advanced prostate cancer
Classification
Peptide and derivative Hormone and analogue
Structure Information
Molecular Formula C70H94N18O16
Molecular Weight 1323.5
Active Sequence XHWSYxLRP
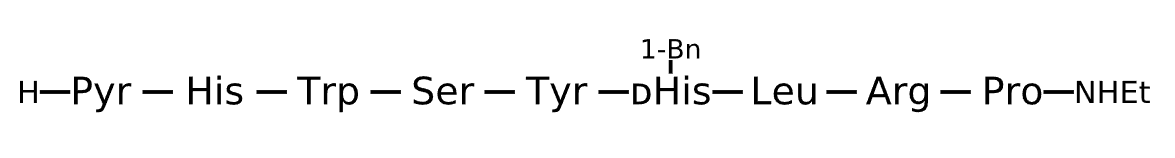
Sequence Length 9
Modification X=Pyr, x=D-His(1-Bn), N-terminal NHEt
IUPAC Name (2S)-N-[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2R)-3-(1-benzylimidazol-4-yl)-1-[[(2S)-1-[[(2S)-5-(diaminomethylideneamino)-1-[(2S)-2-(ethylcarbamoyl)pyrrolidin-1-yl]-1-oxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-1-oxopropan-2-yl]amino]-3-(4-hydroxyphenyl)-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-3-(1H-imidazol-5-yl)-1-oxopropan-2-yl]-5-oxopyrrolidine-2-carboxamide
InChI InChI=1S/C66H86N18O12/c1-4-70-64(95)55-17-11-25-84(55)65(96)48(16-10-24-71-66(67)68)76-58(89)49(26-38(2)3)77-62(93)53(30-43-34-83(37-74-43)33-40-12-6-5-7-13-40)81-59(90)50(27-39-18-20-44(86)21-19-39)78-63(94)54(35-85)82-60(91)51(28-41-31-72-46-15-9-8-14-45(41)46)79-61(92)52(29-42-32-69-36-73-42)80-57(88)47-22-23-56(87)75-47/h5-9,12-15,18-21,31-32,34,36-38,47-55,72,85-86H,4,10-11,16-17,22-30,33,35H2,1-3H3,(H,69,73)(H,70,95)(H,75,87)(H,76,89)(H,77,93)(H,78,94)(H,79,92)(H,80,88)(H,81,90)(H,82,91)(H4,67,68,71)/t47-,48-,49-,50-,51-,52-,53+,54-,55-/m0/s1
InChI_Key HHXHVIJIIXKSOE-QILQGKCVSA-N
SMILES O=C([C@H](CC1)NC1=O)N[C@@H](CC2=CN=CN2)C(N[C@@H](CC3=CNC4=C3C=CC=C4)C(N[C@@H](CO)C(N[C@@H](CC5=CC=C(O)C=C5)C(N[C@H](CC6=CN(CC7=CC=CC=C7)C=N6)C(N[C@@H](CC(C)C)C(N[C@@H](CCC/N=C(N)\N)C(N8[C@H](C(NCC)=O)CCC8)=O)=O)=O)=O)=O)=O)=O
External Codes
PubChem CID 25077993
DrugBank Accession Number DB06788
NCI Thesaurus Code C74270
UNII H50H3S3W74 GSRS
CAS 76712-82-8
Drug approval
Drug indication
As the product Supprelin LA (FDA), histrelin is indicated for the treatment of children with central precocious puberty (CPP). As the product Vantas (FDA), histrelin is indicated for the palliative treatment of advanced prostate cancer.
| Drug Name | Strength | Dosage Form/Route | Company | Marketing Status | Drug ID | Approval year |
|---|---|---|---|---|---|---|
| Supprelin | eq 0.2mg base/ml | Injectable; Injection | Shire | Discontinued | NDA: 019836 | 1991 |
| Supprelin | eq 0.5mg base/ml | Injectable; Injection | Shire | Discontinued | NDA: 019836 | 1991 |
| Supprelin | eq 1mg base/ml | Injectable; Injection | Shire | Discontinued | NDA: 019836 | 1991 |
| Supprelin La | 50mg | Implant; Subcutaneous | Endo Pharm | Prescription | NDA: 022058 | 2007 |
| Vantas | 50mg | Implant; Subcutaneous | Endo Pharm | Discontinued | NDA: 021732 | 2004 |
| Vantas | 50 mg | Implant, Kit; Subcutaneous | Paladin Labs Inc. | Cancelled | DIN: 02278383 | 2006 |
| ClinicalTrials.gov Identifier | Title | Condition or disease | Phase | Purpose |
|---|---|---|---|---|
| NCT01394263 | Phase III, Open-Label Randomized, Parallel, Active-Control Study to Evaluate Efficacy and Safety of Histrelin Subdermal Implant in Patients With Metastatic Prostate Cancer | Prostate Cancer; Adenocarcinoma of the Prostate | Phase 3 | Treatment |
| NCT01574846 | Prospective, Multicentre, Non-interventional (Observational) Study of VANTAS® for the Treatment of Patients With Advanced Prostate Cancer. | Prostate Cancer | Treatment | |
| NCT04513717 | Parallel Phase III Randomized Trials for High Risk Prostate Cancer Evaluating De-Intensification for Lower Genomic Risk and Intensification of Concurrent Therapy for Higher Genomic Risk With Radiation (PREDICT-RT*) | Metastatic Malignant Neoplasm in the Bone; Prostate Adenocarcinoma; Stage III Prostate Cancer AJCC v8; Stage IIIA Prostate Cancer AJCC v8; Stage IIIB Prostate Cancer AJCC v8; Stage IIIC Prostate Cancer AJCC v8; Stage IVA Prostate Cancer AJCC v8 | Phase 3 | Treatment |
| NCT05050084 | Parallel Phase III Randomized Trials of Genomic-Risk Stratified Unfavorable Intermediate Risk Prostate Cancer: De-Intensification and Intensification Clinical Trial Evaluation (GUIDANCE) | Prostate Adenocarcinoma | Phase 3 | Treatment |
| NCT00779103 | Phase III, Open-Label Study to Evaluate Efficacy and Safety of Histrelin Subdermal Implant in Children With Central Precocious Puberty | Central Precocious Puberty | Phase 3 | Treatment |
More clinical information is obtained from ClinicalTrials.gov.