Leuprolide
DRACPC ID DRACPC0019
Active Ingredients Leuprolide
Description A synthetic nonapeptide analogue of gonadotropin-releasing hormone. Leuprolide binds to and activates gonadotropin-releasing hormone (GnRH) receptors. Continuous, prolonged administration of leuprolide in males results in pituitary GnRH receptor desensitization and inhibition of pituitary secretion of follicle stimulating hormone (FSH) and luteinizing hormone (LH), leading to a significant decline in testosterone production; in females, prolonged administration results in a decrease in estradiol production. This agent reduces testosterone production to castration levels and may inhibit androgen receptor-positive tumor progression.
Synonyms Leuprorelin; 6-D-Leucine-9-(N-ethyl-L-prolinamide)-1-9-luteinizing Hormone-releasing Factor (Pig); 6-D-Leucine-9-(N-ethyl-L-prolinamide)-10-deglycinamide Luteinizing Hormone-Releasing Factor (Pig); Leuprolide
Type Small Molecule
Disease Advanced Prostate Cancer, Anemia, Central Precocious Puberty (CPP), Endometriosis
Classification
Peptide and derivative Hormone and analogue
Structure Information
Molecular Formula C59H84N16O12
Molecular Weight 1209.4
Active Sequence XHWSYlLRP
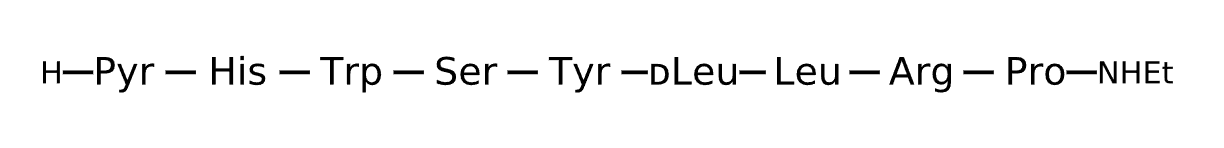
Sequence Length 9
Modification X=Pyr, N-terminal NHEt
IUPAC Name (2S)-N-[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2R)-1-[[(2S)-1-[[(2S)-5-(diaminomethylideneamino)-1-[(2S)-2-(ethylcarbamoyl)pyrrolidin-1-yl]-1-oxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-(4-hydroxyphenyl)-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-3-(1H-imidazol-5-yl)-1-oxopropan-2-yl]-5-oxopyrrolidine-2-carboxamide
InChI InChI=1S/C59H84N16O12/c1-6-63-57(86)48-14-10-22-75(48)58(87)41(13-9-21-64-59(60)61)68-51(80)42(23-32(2)3)69-52(81)43(24-33(4)5)70-53(82)44(25-34-15-17-37(77)18-16-34)71-56(85)47(30-76)74-54(83)45(26-35-28-65-39-12-8-7-11-38(35)39)72-55(84)46(27-36-29-62-31-66-36)73-50(79)40-19-20-49(78)67-40/h7-8,11-12,15-18,28-29,31-33,40-48,65,76-77H,6,9-10,13-14,19-27,30H2,1-5H3,(H,62,66)(H,63,86)(H,67,78)(H,68,80)(H,69,81)(H,70,82)(H,71,85)(H,72,84)(H,73,79)(H,74,83)(H4,60,61,64)/t40-,41-,42-,43+,44-,45-,46-,47-,48-/m0/s1
InChI_Key GFIJNRVAKGFPGQ-LIJARHBVSA-N
SMILES O=C([C@H](CC1)NC1=O)N[C@@H](CC2=CN=CN2)C(N[C@@H](CC3=CNC4=C3C=CC=C4)C(N[C@@H](CO)C(N[C@@H](CC5=CC=C(O)C=C5)C(N[C@H](CC(C)C)C(N[C@@H](CC(C)C)C(N[C@@H](CCC/N=C(N)\N)C(N6[C@H](C(NCC)=O)CCC6)=O)=O)=O)=O)=O)=O)=O
External Codes
PubChem CID 657181
DrugBank Accession Number DB00007
NCI Thesaurus Code C62042
UNII EFY6W0M8TG GSRS
CAS 53714-56-0
Drug approval
Drug indication
Leuprolide is indicated for the treatment of advanced prostate cancer and as palliative treatment of advanced prostate cancer.
It is also used for the treatment of pediatric patients with central precocious puberty (CPP).
In combination with oral norethisterone (also known as norethindrone), leuprolide is also indicated for the initial treatment of the symptoms of endometriosis. Finally, in combination with iron supplementation, leuprolide is indicated for the preoperative hematological improvement of anemic patients with uterine leiomyomata (uterine fibroids).
| Drug Name | Strength | Dosage Form/Route | Company | Marketing Status | Drug ID | Approval year |
|---|---|---|---|---|---|---|
| Camcevi Kit | eq 42mg base | Emulsion; Subcutaneous | Accord | Prescription | NDA: 211488 | 2021 |
| Eligard Kit | 7.5mg | Powder; Subcutaneous | Tolmar Therap | Prescription | NDA: 021343 | 2002 |
| Eligard Kit | 22.5mg | Powder; Subcutaneous | Tolmar Therap | Prescription | NDA: 021379 | 2002 |
| Eligard Kit | 30mg | Powder; Subcutaneous | Tolmar Therap | Prescription | NDA: 021488 | 2003 |
| Eligard Kit | 45mg | Powder; Subcutaneous | Tolmar Therap | Prescription | NDA: 021731 | 2004 |
| Fensolvi Kit | 45mg | Powder; Subcutaneous | Tolmar | Prescription | NDA: 213150 | 2020 |
| Leuprolide Acetate | 1mg/0.2ml | Injectable; Injection | Sandoz | Prescription | ANDA: 074728 | 1998 |
| Leuprolide Acetate | 1mg/0.2ml | Injectable; Injection | Teva Pharms Usa | Prescription | ANDA: 075471 | 2000 |
| Leuprolide Acetate | 1mg/0.2ml | Injectable; Injection | Genzyme | Prescription | ANDA: 075721 | 2001 |
| Leuprolide Acetate | 1mg/0.2ml | Injectable; Injection | Sun Pharm | Prescription | ANDA: 078885 | 2009 |
| Leuprolide Acetate | 1mg/0.2ml | Injectable; Injection | Eugia Pharma | Prescription | ANDA: 212963 | 2022 |
| Leuprolide Acetate | 1mg/0.2ml | Injectable; Injection | Vgyaan | Prescription | ANDA: 213829 | 2021 |
| Lupaneta Pack | 3.75mg/vial, n/a; n/a, 5mg | Leuprolide Acetate; Norethindrone Acetate | Abbvie Endocrine | Discontinued | NDA: 203696 | 2012 |
| Lupaneta Pack | 11.25mg/vial ,n/a; n/a, 5mg | Leuprolide Acetate; Norethindrone Acetate | Abbvie Endocrine | Discontinued | NDA: 203696 | 2012 |
| Lupron | 1mg/0.2ml | Injectable; Injection | Abbvie Endocrine Inc | Discontinued | NDA: 019010 | 1985 |
| Lupron Depot | 7.5mg/vial | Injectable; Injection | Abbvie Endocrine Inc | Prescription | NDA: 019732 | 1989 |
| Lupron Depot | 3.75mg | Injectable; Injection | Abbvie Endocrine Inc | Prescription | NDA: 019943 | 1995 |
| Lupron Depot | 3.75mg/vial | Injectable; Injection | Abbvie Endocrine Inc | Discontinued | NDA: 020011 | 1990 |
| Lupron Depot | 3.75mg | Injectable; Injection | Abbvie Endocrine Inc | Prescription | NDA: 020011 | 1990 |
| Lupron Depot | 22.5mg/vial | Injectable; Injection | Abbvie Endocrine Inc | Prescription | NDA: 020517 | 1995 |
| Lupron Depot | 30mg/vial | Injectable; Injection | Abbvie Endocrine Inc | Prescription | NDA: 020517 | 1995 |
| Lupron Depot | 45mg/vial | Injectable; Injection | Abbvie Endocrine Inc | Prescription | NDA: 020517 | 1995 |
| Lupron Depot | 11.25mg/vial | Injectable; Injection | Abbvie Endocrine Inc | Prescription | NDA: 020708 | 1997 |
| Lupron Depot-Ped Kit | 7.5mg | Powder; Intramuscular | Abbvie Endocrine Inc | Prescription | NDA: 020263 | 1993 |
| Lupron Depot-Ped Kit | 3.75mg, 7.5mg | Powder; Intramuscular | Abbvie Endocrine Inc | Discontinued | NDA: 020263 | 1993 |
| Lupron Depot-Ped Kit | 7.5mg, 7.5mg | Powder; Intramuscular | Abbvie Endocrine Inc | Discontinued | NDA: 020263 | 1993 |
| Lupron Depot-Ped Kit | 11.25mg | Powder; Intramuscular | Abbvie Endocrine Inc | Prescription | NDA: 020263 | 1993 |
| Lupron Depot-Ped Kit | 15mg | Powder; Intramuscular | Abbvie Endocrine Inc | Prescription | NDA: 020263 | 1993 |
| Lupron Depot-Ped Kit | 11.25mg | Powder; Intramuscular | Abbvie Endocrine Inc | Prescription | NDA: 020263 | 1993 |
| Lupron Depot-Ped Kit | 30mg | Powder; Intramuscular | Abbvie Endocrine Inc | Prescription | NDA: 020263 | 1993 |
| Lutrate Depot Kit | 22.5mg/vial | For Suspension; Intramuscular | Gp-Pharm Sa | Prescription | NDA: 205054 | 2018 |
| Viadur | eq 65mg base | Implant; Implantation | Ortho Mcneil Janssen | Discontinued | NDA: 021088 | 2000 |
| Camcevi | unknown | Prolonged-Release Suspension; Subcutaneous | Accord Healthcare S.L.U. | Prescription | EMEA/H/C/005034 | 2022 |
| Camcevi | 42 mg / syr | Emulsion(Extended-Release); Subcutaneous | Accord Healthcare Inc | Prescription | DIN: 02522446 | 2021 |
| Eligard | 7.5 mg / syr | Powder For Suspension(Sustained-Release); Subcutaneous | Tolmar International Ltd. | Prescription | DIN: 02248239 | 2003 |
| Eligard | 22.5 mg / syr | Powder For Suspension(Sustained-Release); Subcutaneous | Tolmar International Ltd. | Prescription | DIN: 02248240 | 2003 |
| Eligard | 30 mg / syr | Powder For Suspension(Sustained-Release); Subcutaneous | Tolmar International Ltd. | Prescription | DIN: 02248999 | 2004 |
| Eligard | 45 mg / syr | Powder For Suspension(Sustained-Release); Subcutaneous | Tolmar International Ltd. | Prescription | DIN: 02268892 | 2005 |
| Leuprolide Acetate Injection | 5 mg / ml | Liquid; Subcutaneous | Novopharm Limited | Cancelled | DIN: 02240264 | 2015 |
| Lupron | 5 mg / ml | Solution; Subcutaneous | Abbvie Corporation | Prescription | DIN: 00727695 | 1985 |
| Lupron Depot | 7.5 mg / syr | Powder For Suspension(Sustained-Release); Intramuscular | Abbvie Corporation | Prescription | DIN: 00836273 | 1989 |
| Lupron Depot | 3.75 mg / syr | Powder For Suspension(Sustained-Release); Intramuscular | Abbvie Corporation | Prescription | DIN: 00884502 | 1992 |
| Lupron Depot | 11.25 mg / vial | Powder For Suspension(Sustained-Release); Intramuscular | Abbvie Corporation | Cancelled | DIN: 02148722 | 2016 |
| Lupron Depot | 15 mg / vial | Powder For Suspension(Sustained-Release); Intramuscular | Abbvie Corporation | Cancelled | DIN: 02148730 | 2016 |
| Lupron Depot | 22.5 mg / syr | Powder For Suspension(Sustained-Release); Intramuscular | Abbvie Corporation | Prescription | DIN: 02230248 | 1997 |
| Lupron Depot | 30 mg / syr | Powder For Suspension(Sustained-Release); Intramuscular | Abbvie Corporation | Prescription | DIN: 02239833 | 1999 |
| Lupron Depot | 11.25 mg / syr | Powder For Suspension(Sustained-Release); Intramuscular | Abbvie Corporation | Prescription | DIN: 02239834 | 1999 |
| Zeulide Depot | 3.75 mg / vial | Powder For Suspension(Sustained-Release), Kit; Intramuscular | Verity Pharmaceuticals Inc. | Prescription | DIN: 02429977 | 2019 |
| Zeulide Depot | 22.5 mg / vial | Powder For Suspension(Sustained-Release), Kit; Intramuscular | Verity Pharmaceuticals Inc. | Prescription | DIN: 02462699 | 2019 |
| ClinicalTrials.gov Identifier | Title | Condition or disease | Phase | Purpose |
|---|---|---|---|---|
| NCT03035032 | A Phase IV Interventional Safety Study of ELIGARD® in Prostate Cancer Patients in Asia (ELIGANT) | Prostate Cancer | Phase 4 | Treatment |
| NCT02319837 | A Phase 3, Randomized, Efficacy and Safety Study of Enzalutamide Plus Leuprolide, Enzalutamide Monotherapy, and Placebo Plus Leuprolide in Men With High-Risk Nonmetastatic Prostate Cancer Progressing After Definitive Therapy | Hormone Sensitive Prostate Cancer; Prostate Cancer; Cancer of the Prostate | Phase 3 | Treatment |
| NCT02508636 | Phase II Trial of Definitive Radiotherapy With Leuprolide and Enzalutamide in High Risk Prostate Cancer | Prostatic Neoplasms; Pelvic Nodal | Phase 2 | Treatment |
| NCT00254397 | Study of the Modulatory Activity of an LHRH-Agonist (Leuprolide) on Melanoma Peptide Vaccines as Adjuvant Therapy in Melanoma Patients | Melanoma | Phase 2 | Treatment |
| NCT00903162 | Extended Endocrine Therapy for Premenopausal Women With Breast Cancer | Breast Cancer | Phase 2 | Treatment |
More clinical information is obtained from ClinicalTrials.gov.