ADH-1
DRACPC ID DRACPC0030
Active Ingredients ADH-1
Description A small, cyclic pentapeptide vascular-targeting agent with potential antineoplastic and antiangiogenic activities. ADH-1 selectively and competitively binds to and blocks N-cadherin, which may result in disruption of tumor vasculature, inhibition of tumor cell growth, and the induction of tumor cell and endothelial cell apoptosis. N-cadherin, a cell- surface transmembrane glycoprotein of the cadherin superfamily of proteins involved in calcium-mediated cell-cell adhesion and signaling mechanisms; may be upregulated in some aggressive tumors and the endothelial cells and pericytes of some tumor blood vessels.
Synonyms Exherin; ADH-1
Type Biotech
Disease Cancer/Tumor
Classification
Peptide and derivative Cyclic Antiangiogenesis agent
Structure Information
Molecular Formula C22H34N8O6S2
Molecular Weight 570.7
Active Sequence CHAVC
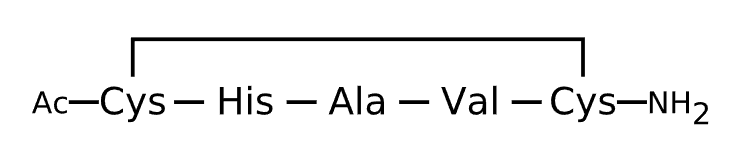
Sequence Length 5
Modification C-terminal Ac, N-terminal NH2
IUPAC Name (4R,7S,10S,13S,16R)-16-acetamido-13-(1H-imidazol-5-ylmethyl)-10-methyl-6,9,12,15-tetraoxo-7-propan-2-yl-1,2-dithia-5,8,11,14-tetrazacycloheptadecane-4-carboxamide
InChI InChI=1S/C22H34N8O6S2/c1-10(2)17-22(36)29-15(18(23)32)7-37-38-8-16(27-12(4)31)21(35)28-14(5-13-6-24-9-25-13)20(34)26-11(3)19(33)30-17/h6,9-11,14-17H,5,7-8H2,1-4H3,(H2,23,32)(H,24,25)(H,26,34)(H,27,31)(H,28,35)(H,29,36)(H,30,33)/t11-,14-,15-,16-,17-/m0/s1
InChI_Key FQVLRGLGWNWPSS-BXBUPLCLSA-N
SMILES O=C(C)N[C@H](C(N[C@H](C(N[C@H](C(N[C@H](C(N1)=O)C(C)C)=O)C)=O)CC2=CN=CN2)=O)CSSC[C@H]1C(N)=O
External Codes
PubChem CID 9916058
DrugBank Accession Number DB05485
NCI Thesaurus Code C53399
UNII B058ME29VU GSRS
CAS 229971-81-7
Drug approval
Drug indication
Investigated for use/treatment in breast cancer, cancer/tumors (unspecified), melanoma, ovarian cancer, and solid tumors.
The drug is not approved.
| ClinicalTrials.gov Identifier | Title | Condition or disease | Phase | Purpose |
|---|---|---|---|---|
| NCT00421811 | An Open-Label, Multicenter, Phase 1/2 Dose Escalation Study to Evaluate Safety, Tolerability and Anti-tumor Activity of Systemic ADH-1 in Combination With Normothermic Isolated Limb Infusion of Melphalan in Subjects With Locally Advanced In-Transit Malignant Melanoma (Adherex Protocol Number AHX-01-007). | Neoplasms | Phase 1/2 | Treatment |
| NCT00265057 | Dose Finding, Safety, Pharmacokinetic and Pharmacodynamic Study of the Vascular Targeting Agent Exherin™ (ADH-1) Administered Once Weekly in 3 Week Cycles by Intravenous Infusion in Patients With N-Cadherin Expressing, Incurable, Solid Tumors (Adherex Protocol Number AHX-01-004) | Neoplasms | Phase 1/2 | Treatment |
| NCT00264433 | An Open-Label Phase 2a Study in Subjects With N-Cadherin Positive, Advanced or Recurrent Solid Tumors to Investigate the Safety and Efficacy of ADH-1 Administered Intravenously as a Single Agent (Adherex Protocol Number AHX-01-201) | Neoplasms | Phase 2 | Treatment |
| NCT00390676 | A Phase 1, Multicenter, Dose-Escalation Study to Investigate the Safety and Tolerability of ADH-1 in Combination With 1) Carboplatin or 2) Docetaxel or 3) Capecitabine in Subjects With N-Cadherin Positive, Advanced Solid Tumors (Adherex Protocol Number AHX-01-006) | Neoplasms | Phase 1 | Treatment |
| NCT00225550 | Dose Finding, Safety, Pharmacokinetic and Pharmacodynamic Study of the Vascular Targeting Agent Exherin™ (ADH-1) Administered Once Daily for 5 Consecutive Days by Intravenous Infusion in Subjects With N-Cadherin Expressing, Incurable, Solid Tumors (Adherex Protocol Number AHX 01 003) | Neoplasms | Phase 1/2 | Treatment |
More clinical information is obtained from ClinicalTrials.gov.