Canfosfamide
DRACPC ID DRACPC0032
Active Ingredients Canfosfamide
Description A modified glutathione analogue and nitrogen mustard prodrug, with potential antineoplastic activity. Canfosfamide is selectively activated by glutathione S-transferase P1-1 into an alkylating metabolite that forms covalent linkages with nucleophilic centers in tumor cell DNA, which may induce a cellular stress response and cytotoxicity, and decrease tumor cell proliferation. Glutathione S-transferase P1-1 is an enzyme that is overexpressed in many human malignancies.
Synonyms (2R)-L-gamma-Glutamyl-3-((2-((bis(bis(2-chloroethyl)amino)phosphinyl)oxy)ethyl)sulfonyl)-L-alanyl-2-phenylglycine; Ter 286; Canfosfamide
Type Small Molecule
Disease Various forms of cancer
Classification
Peptide and derivative
Structure Information
Molecular Formula C26H40Cl4N5O10PS
Molecular Weight 787.5
Active Sequence XXx
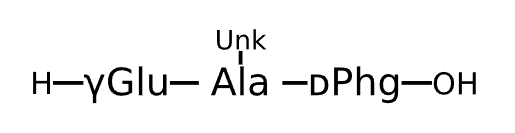
Sequence Length 3
Modification X(1)=gGlu, X(2)=Ala(Unk), x=D-Phg
IUPAC Name (2S)-2-amino-5-[[(2R)-3-[2-[bis[bis(2-chloroethyl)amino]phosphoryloxy]ethylsulfonyl]-1-[[(R)-carboxy(phenyl)methyl]amino]-1-oxopropan-2-yl]amino]-5-oxopentanoic acid
InChI InChI=1S/C26H40Cl4N5O10PS/c27-8-12-34(13-9-28)46(42,35(14-10-29)15-11-30)45-16-17-47(43,44)18-21(32-22(36)7-6-20(31)25(38)39)24(37)33-23(26(40)41)19-4-2-1-3-5-19/h1-5,20-21,23H,6-18,31H2,(H,32,36)(H,33,37)(H,38,39)(H,40,41)/t20-,21-,23+/m0/s1
InChI_Key OJLHWPALWODJPQ-QNWVGRARSA-N
SMILES O=C(O)[C@@H](N)CCC(N[C@@H](CS(=O)(CCOP(N(CCCl)CCCl)(N(CCCl)CCCl)=O)=O)C(N[C@@H](C(O)=O)C1=CC=CC=C1)=O)=O
External Codes
PubChem CID 5312109
DrugBank Accession Number DB04972
NCI Thesaurus Code C83581
UNII 1RS284BFUI GSRS
CAS 158382-37-7
Drug approval
Drug indication
Intended for the treatment of various forms of cancer.
The drug is not approved.
| ClinicalTrials.gov Identifier | Title | Condition or disease | Phase | Purpose |
|---|---|---|---|---|
| NCT01148108 | Phase 2 Study of Canfosfamide HCl for Injection (Telcyta®, TLK286)in Refractory or Relapsed Mantle Cell Lymphoma (MCL), Diffuse Large B Cell Lymphoma (DLBCL) and Multiple Myeloma (MM) | Mantle Cell Lymphoma; B Cell Lymphoma; Multiple Myeloma | Phase 2 | Treatment |
| NCT00022347 | A Phase II Study of TLK 286 in Platinum Resistant Advanced Epithelial Ovarian Cancer | Fallopian Tube Cancer; Ovarian Cancer; Primary Peritoneal Cavity Cancer | Phase 2 | Treatment |
| NCT00036920 | Phase II Study Of TLK286 For The Treatment Of Advanced Non-Small Cell Lung Cancer | Lung Cancer | Phase 2 | Treatment |
| NCT00350948 | Phase 3 Randomized Study of TLK286 (Telcyta®) in Combination With Liposomal Doxorubicin (Doxil/Caelyx)Versus Liposomal Doxorubicin (Doxil/Caelyx) as Second-Line Therapy in Platinum Refractory or Resistant Ovarian Cancer (ASSIST-5) | Ovarian Neoplasms | Phase 3 | Treatment |
More clinical information is obtained from ClinicalTrials.gov.