Darinaparsin
DRACPC ID DRACPC0034
Active Ingredients Darinaparsin
Description A small-molecule organic arsenical with potential antineoplastic activity. Although the exact mechanism of action is unclear, darinaparsin, a highly toxic metabolic intermediate of inorganic arsenicals (iAs) that occurs in vivo, appears to generate volatile cytotoxic arsenic compounds when glutathione (GSH) concentrations are low. The arsenic compounds generated from darinaparsin disrupt mitochondrial bioenergetics, producing reactive oxygen species (ROS) and inducing ROS-mediated tumor cell apoptosis; in addition, this agent or its byproducts may initiate cell death by interrupting the G2/M phase of the cell cycle and may exhibit antiangiogenic effects. Compared to inorganic arsenic compounds such as arsenic trioxide (As2O3), darinaparsin appears to exhibit a wide therapeutic window.
Synonyms DMAIII(SG); S-Dimethylarsino-Glutathione; ZIO-101; Darinaparsin
Type Small Molecule
Disease Cancer/Tumor
Classification
Peptide and derivative
Structure Information
Molecular Formula C12H22AsN3O6S
Molecular Weight 411.31
Active Sequence XXG
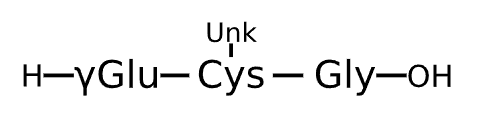
Sequence Length 3
Modification X(1)=gGlu, X(2)=Cys(Unk)
IUPAC Name (2S)-2-amino-5-[[(2R)-1-(carboxymethylamino)-3-dimethylarsanylsulfanyl-1-oxopropan-2-yl]amino]-5-oxopentanoic acid
InChI InChI=1S/C12H22AsN3O6S/c1-13(2)23-6-8(11(20)15-5-10(18)19)16-9(17)4-3-7(14)12(21)22/h7-8H,3-6,14H2,1-2H3,(H,15,20)(H,16,17)(H,18,19)(H,21,22)/t7-,8-/m0/s1
InChI_Key JGDXFQORBMPJGR-YUMQZZPRSA-N
SMILES O=C(O)[C@@H](N)CCC(N[C@@H](CS[As](C)C)C(NCC(O)=O)=O)=O
External Codes
PubChem CID 11683005
DrugBank Accession Number DB06179
NCI Thesaurus Code C61490
UNII 9XX54M675G GSRS
CAS 69819-86-9
Drug approval
Drug indication
Investigated for use/treatment in blood (blood forming organ disorders, unspecified), cancer/tumors (unspecified), solid tumors, multiple myeloma, and liver cancer.
The drug is not approved.
| ClinicalTrials.gov Identifier | Title | Condition or disease | Phase | Purpose |
|---|---|---|---|---|
| NCT01139359 | A Phase I Dose Escalation Study of Darinaparsin in Combination With CHOP in Previously Untreated Patients With Lymphomas Who Are Scheduled to Receive CHOP Alone | Lymphoma | Phase 1 | Treatment |
| NCT01139346 | Phase I Study of Oral Darinaparsin in Advanced Solid Tumors | Advanced Solid Tumors | Phase 1 | Treatment |
| NCT00421213 | Phase II Study of ZIO-101 in Advanced Blood and Bone Marrow Cancers | Hematologic Neoplasms; Bone Marrow Neoplasms; Non-Hodgkin's Lymphoma | Phase 2 | Treatment |
| NCT00423306 | A Phase-II Trial of ZIO 101 in Advanced Hepatocellular Carcinoma | Hepatocellular Carcinoma | Phase 2 | Treatment |
| NCT00509782 | Phase I Trial of ZIO-101 in Patients With Solid Tumors | Solid Tumors | Phase 1 | Treatment |
More clinical information is obtained from ClinicalTrials.gov.