LCL-161
DRACPC ID DRACPC0038
Active Ingredients LCL-161
Description An orally bioavailable second mitochondrial-derived activator of caspases (SMAC) mimetic and inhibitor of IAP (Inhibitor of Apoptosis Protein) family of proteins, with potential antineoplastic activity. SMAC mimetic LCL161 binds to IAPs, such as X chromosome-linked IAP (XIAP) and cellular IAPs 1 and 2. Since IAPs shield cancer cells from the apoptosis process, this agent may restore and promote the induction of apoptosis through apoptotic signaling pathways in cancer cells. IAPs are overexpressed by many cancer cell types and suppress apoptosis by binding and inhibiting active caspases-3, -7 and -9, which play essential roles in apoptosis (programmed cell death), necrosis and inflammation.
Synonyms Smac Mimetic LCL161; LCL-161
Type Small Molecule
Disease Leukemia, Neoplasms, Solid Tumors, Breast Cancer, Ovarian Cancer
Classification
Inhibitor of IAP family of proteins Peptide and derivative
Structure Information
Molecular Formula C26H33FN4O3S
Molecular Weight 500.6
Active Sequence AX
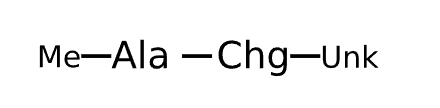
Sequence Length 2
Modification X=Chg-Unk, C-terminal Me
IUPAC Name (2S)-N-[(1S)-1-cyclohexyl-2-[(2S)-2-[4-(4-fluorobenzoyl)-1,3-thiazol-2-yl]pyrrolidin-1-yl]-2-oxoethyl]-2-(methylamino)propanamide
InChI InChI=1S/C26H33FN4O3S/c1-16(28-2)24(33)30-22(17-7-4-3-5-8-17)26(34)31-14-6-9-21(31)25-29-20(15-35-25)23(32)18-10-12-19(27)13-11-18/h10-13,15-17,21-22,28H,3-9,14H2,1-2H3,(H,30,33)/t16-,21-,22-/m0/s1
InChI_Key UFPFGVNKHCLJJO-SSKFGXFMSA-N
SMILES C[C@H](NC)C(N[C@@H](C1CCCCC1)C(N2[C@H](C3=NC(C(C4=CC=C(F)C=C4)=O)=CS3)CCC2)=O)=O
External Codes
PubChem CID 24737642
DrugBank Accession Number DB12085
NCI Thesaurus Code C91079
UNII 6TNS415Y3P GSRS
CAS 1005342-46-0
Drug approval
Drug indication
LCL161 has been used in trials studying the treatment of Leukemia, Neoplasms, Solid Tumors, Breast Cancer, and Ovarian Cancer, among others.
The drug is not approved.
| ClinicalTrials.gov Identifier | Title | Condition or disease | Phase | Purpose |
|---|---|---|---|---|
| NCT01968915 | A Phase I Study of Oral LCL161 in Japanese Adult Patients With Advanced Solid Tumors | Neoplasms | Phase 1 | Treatment |
| NCT01955434 | Phase II Study of LCL161 Alone and in Combination With Cyclophosphamide in Patients With Relapsed or Refractory Multiple Myeloma | Recurrent Plasma Cell Myeloma; Refractory Plasma Cell Myeloma | Phase 2 | Treatment |
| NCT02098161 | Open Label Phase 2 Single Agent Study of LCL-161 in Patients With Primary Myelofibrosis (PMF), Post-Polycythemia Vera Myelofibrosis (Post-PV MF), or Post-Essential Thrombocytosis Myelofibrosis (Post-ET MF) | Polycythemia Vera, Post-Polycythemic Myelofibrosis Phase; Primary Myelofibrosis; Secondary Myelofibrosis | Phase 2 | Treatment |
| NCT01934634 | Phase I Trial of the Proapoptotic Agonist, LCL161, and Gemcitabine Plus Nab-Paclitaxel in Patients With Metastatic Pancreatic Cancer | Metastatic Pancreatic Cancer | Phase 1 | Treatment |
| NCT01240655 | A Phase Ib Study of LCL161 in Combination With Weekly Paclitaxel in Adult Patients With Advanced Solid Tumors | Solid Tumors | Phase 1 | Treatment |
More clinical information is obtained from ClinicalTrials.gov.