Tigapotide
DRACPC ID DRACPC0041
Active Ingredients Tigapotide
Description A synthetic 15-mer peptide corresponding to amino acids 31-45 of the 94-amino acid isoform of human prostate secretory protein (PSP-94) with potential anti-metastasis and anti-angiogenesis activities. PSP-94-derived peptide PCK3145 may inhibit the secretion of the metastasis-related protein matrix metalloproteinase-9 (MMP-9) and its potential binding to its cell surface receptor CD44; may interfere with the vascular endothelial growth factor (VEGF) signaling pathway, resulting in an anti-angiogenesis effect; and may reduce the levels of parathyroid hormone-related protein (PTHrP), decreasing plasma calcium levels. PSP-94, one of three predominant proteins found in seminal fluid, may be down-regulated in prostate cancer, representing a potential survival mechanism for prostate cancer cells. MMP-9 is implicated in the invasion and metastasis of cancer. PTHrP may be expressed by various tumor cell types, resulting in the hypercalcemia of malignancy.
Synonyms PCK3145; Prostate-Secretory Protein-94-Derived Peptide PCK3145; Tigapotide
Type Biotech
Disease Hormone Refractory Prostate Cancer (HRPC)
Classification
Peptide and derivative Antiangiogenesis agent
Structure Information
Molecular Formula C82H119N21O34S3
Molecular Weight 2039.1
Active Sequence EWQTDNXETXTXYET
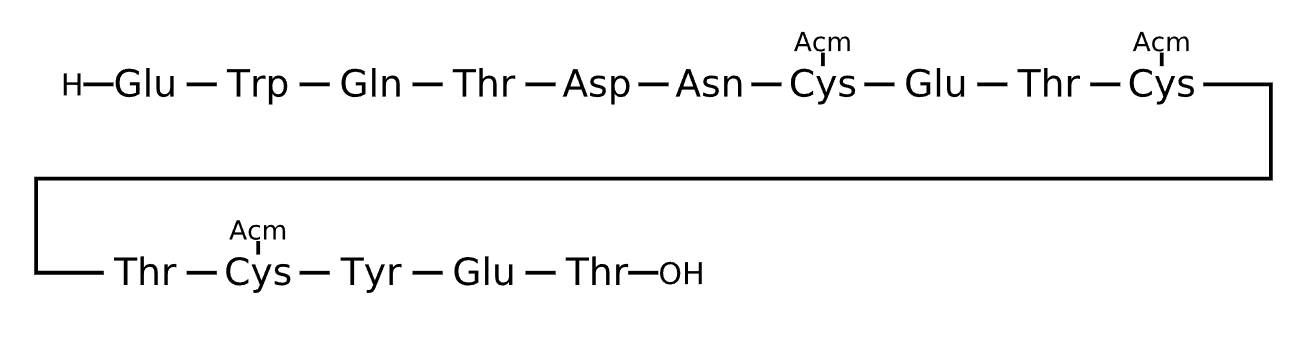
Sequence Length 15
Modification X=Cys(ACM)
IUPAC Name (4S)-5-[[(2S)-1-[[(2S)-1-[[(2S,3R)-1-[[(2S)-1-[[(2S)-1-[[(2R)-3-(acetamidomethylsulfanyl)-1-[[(2S)-1-[[(2S,3R)-1-[[(2R)-3-(acetamidomethylsulfanyl)-1-[[(2S,3R)-1-[[(2R)-3-(acetamidomethylsulfanyl)-1-[[(2S)-1-[[(2S)-4-carboxy-1-[[(1S,2R)-1-carboxy-2-hydroxypropyl]amino]-1-oxobutan-2-yl]amino]-3-(4-hydroxyphenyl)-1-oxopropan-2-yl]amino]-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxobutan-2-yl]amino]-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxobutan-2-yl]amino]-4-carboxy-1-oxobutan-2-yl]amino]-1-oxopropan-2-yl]amino]-4-amino-1,4-dioxobutan-2-yl]amino]-3-carboxy-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxobutan-2-yl]amino]-5-amino-1,5-dioxopentan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-4-amino-5-oxopentanoic acid
InChI InChI=1S/C82H119N21O34S3/c1-35(104)64(100-69(123)48(17-20-58(84)112)90-73(127)52(93-68(122)46(83)16-21-60(114)115)25-43-28-86-47-11-9-8-10-45(43)47)79(133)96-54(27-63(120)121)75(129)95-53(26-59(85)113)74(128)97-55(29-138-32-87-39(5)108)76(130)92-49(18-22-61(116)117)70(124)101-65(36(2)105)80(134)99-57(31-140-34-89-41(7)110)78(132)102-66(37(3)106)81(135)98-56(30-139-33-88-40(6)109)77(131)94-51(24-42-12-14-44(111)15-13-42)72(126)91-50(19-23-62(118)119)71(125)103-67(38(4)107)82(136)137/h8-15,28,35-38,46,48-57,64-67,86,104-107,111H,16-27,29-34,83H2,1-7H3,(H2,84,112)(H2,85,113)(H,87,108)(H,88,109)(H,89,110)(H,90,127)(H,91,126)(H,92,130)(H,93,122)(H,94,131)(H,95,129)(H,96,133)(H,97,128)(H,98,135)(H,99,134)(H,100,123)(H,101,124)(H,102,132)(H,103,125)(H,114,115)(H,116,117)(H,118,119)(H,120,121)(H,136,137)/t35-,36-,37-,38-,46+,48+,49+,50+,51+,52+,53+,54+,55+,56+,57+,64+,65+,66+,67+/m1/s1
InChI_Key ZRXXHPDJLAQCPC-SFJRRRFZSA-N
SMILES O=C(O)CC[C@H](N)C(N[C@@H](CC1=CNC2=C1C=CC=C2)C(N[C@@H](CCC(N)=O)C(N[C@@H]([C@H](O)C)C(N[C@@H](CC(O)=O)C(N[C@@H](CC(N)=O)C(N[C@@H](CSCNC(C)=O)C(N[C@@H](CCC(O)=O)C(N[C@@H]([C@H](O)C)C(N[C@@H](CSCNC(C)=O)C(N[C@@H]([C@H](O)C)C(N[C@@H](CSCNC(C)=O)C(N[C@@H](CC3=CC=C(O)C=C3)C(N[C@@H](CCC(O)=O)C(N[C@H](C(O)=O)[C@H](O)C)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O
External Codes
PubChem CID 16155604
DrugBank Accession Number DB04985
NCI Thesaurus Code C78474
UNII 1WZ6S45S94 GSRS
CAS 848084-83-3
Drug approval
Drug indication
For the treatment of late stage Hormone Refractory Prostate Cancer (HRPC) for which no effective therapy currently exists.
The drug is not approved.
| ClinicalTrials.gov Identifier | Title | Condition or disease | Phase | Purpose |
|---|---|---|---|---|
| NCT00695851 | A Phase I Randomized Dose-Seeking Trial of PCK3145 in Asymptomatic, Castrate Metastatic Prostate Cancer Patients. | Prostate Cancer | Phase 1 | Treatment |
More clinical information is obtained from ClinicalTrials.gov.