E7974
DRACPC ID DRACPC0048
Active Ingredients E7974
Description An analog of the sponge-derived anti-microtubule tripeptide hemiasterlin with antimitotic and potential antineoplastic activities. Hemiasterlin analog E7974 binds to the Vinca domain on tubulin, resulting in inhibition of tubulin polymerization and microtubule assembly; depolymerization of existing microtubules; inhibition of mitosis; and inhibition of cellular proliferation. This agent may have more affinity for the beta-3 tubulin isotype.
Synonyms E-7974; E7974; Hemiasterlin Analog E7974
Type Small Molecule
Disease Cancer, Malignant Tumors
Classification
Peptide and derivative Marine origin
Structure Information
Molecular Formula C24H43N3O4
Molecular Weight 437.6
Active Sequence Not available
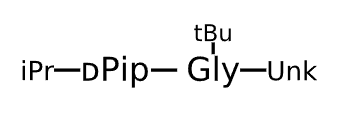
Sequence Length Not available
Modification Not available
IUPAC Name (E,4S)-4-[[(2S)-3,3-dimethyl-2-[[(2R)-1-propan-2-ylpiperidine-2-carbonyl]amino]butanoyl]-methylamino]-2,5-dimethylhex-2-enoic acid
InChI InChI=1S/C24H43N3O4/c1-15(2)19(14-17(5)23(30)31)26(9)22(29)20(24(6,7)8)25-21(28)18-12-10-11-13-27(18)16(3)4/h14-16,18-20H,10-13H2,1-9H3,(H,25,28)(H,30,31)/b17-14+/t18-,19-,20-/m1/s1
InChI_Key FWYMMXUDTATKLG-PGLMLKKXSA-N
SMILES OC(/C(C)=C/[C@H](C(C)C)N(C)C([C@@H](NC([C@@H]1N(CCCC1)C(C)C)=O)C(C)(C)C)=O)=O
External Codes
PubChem CID 10127315
DrugBank Accession Number Not available
NCI Thesaurus Code C49083
UNII 1BO0C11BSH GSRS
CAS 610787-07-0
Drug approval
Drug indication
Investigated for use/treatment in Cancer and Malignant Tumors.
The drug is not approved.
| ClinicalTrials.gov Identifier | Title | Condition or disease | Phase | Purpose |
|---|---|---|---|---|
| NCT00165802 | A Phase I Open Label Study of E7974 Administered on Day 1 of a 21-Day Cycle In Patients With Advanced Solid Tumors | Cancer, Malignant Tumors | Phase 1 | Treatment |
| NCT00130169 | A Phase 1 Open-Label Study of E7974 Administered on Days 1 and 15 of a 28-Day Cycle in Patients With Solid Malignancies | Cancer, Malignant Tumors | Phase 1 | Treatment |
| NCT00121732 | A Phase 1, Two-Arm, Open-Label Study of E7974 Administered on Days 1, 8, and 15 of a 28-Day Cycle and Days 1 and 8 of a 21-Day Cycle in Patients With Solid Malignancies | Cancer, Malignant Tumors | Phase 1 | Treatment |
More clinical information is obtained from ClinicalTrials.gov.