Cilengitide
DRACPC ID DRACPC0053
Active Ingredients Cilengitide
Description A cyclic Arg-Gly-Asp peptide with potential antineoplastic activity. Cilengitide binds to and inhibits the activities of the alpha(v)beta(3) and alpha(v)beta(5) integrins, thereby inhibiting endothelial cell-cell interactions, endothelial cell-matrix interactions, and angiogenesis.
Synonyms cyclo(L-arginylglycyl-L-a-aspartyl-D-phenylalanyl-N-methyl-L-valyl); EMD 121974; EMD-121974; Cilengitide
Type Small Molecule
Disease Sarcoma, Gliomas, Lymphoma, Leukemia, Lung Cancer
Classification
Integrin αvβ3/αvβ5 inhibitor Peptide and derivative Cyclic Antiangiogenesis agent
Structure Information
Molecular Formula C27H40N8O7
Molecular Weight 588.7
Active Sequence RGDfX
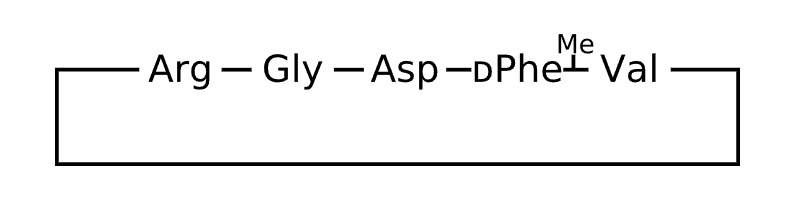
Sequence Length 5
Modification X=N(Me)Val
IUPAC Name 2-[(2S,5R,8S,11S)-5-benzyl-11-[3-(diaminomethylideneamino)propyl]-7-methyl-3,6,9,12,15-pentaoxo-8-propan-2-yl-1,4,7,10,13-pentazacyclopentadec-2-yl]acetic acid
InChI InChI=1S/C27H40N8O7/c1-15(2)22-25(41)33-17(10-7-11-30-27(28)29)23(39)31-14-20(36)32-18(13-21(37)38)24(40)34-19(26(42)35(22)3)12-16-8-5-4-6-9-16/h4-6,8-9,15,17-19,22H,7,10-14H2,1-3H3,(H,31,39)(H,32,36)(H,33,41)(H,34,40)(H,37,38)(H4,28,29,30)/t17-,18-,19+,22-/m0/s1
InChI_Key AMLYAMJWYAIXIA-VWNVYAMZSA-N
SMILES O=C(O)C[C@@H](C(N[C@H](CC1=CC=CC=C1)C(N(C)[C@@H](C(C)C)C(N[C@@H](CCC/N=C(N)\N)C(NC2)=O)=O)=O)=O)NC2=O
External Codes
PubChem CID 176873
DrugBank Accession Number DB11890
NCI Thesaurus Code C1834
UNII 4EDF46E4GI GSRS
CAS 188968-51-6
Drug approval
Drug indication
Cilengitide has been used in trials studying the treatment of Sarcoma, Gliomas, Lymphoma, Leukemia, and Lung Cancer, among others.
The drug is not approved.
| ClinicalTrials.gov Identifier | Title | Condition or disease | Phase | Purpose |
|---|---|---|---|---|
| NCT01517776 | Cilengitide and Metronomic Temozolomide for Relapsed or Refractory High Grade Gliomas or Diffuse Intrinsic Pontine Gliomas in Children and Adolescents - A Phase II Study HIT-HGG-CilMetro - A Clinical Phase II Trial of the HIT-HGG Study Group - | Gliomas | Phase 2 | Treatment |
| NCT01118676 | Phase I Trial Evaluating Continuous Infusion of Cilengitide Together With Radiochemotherapy in Patients With Locally Advanced Non Small Cell Lung Cancer | Locally Advanced Non Small Cell Lung Cancer (NSCLC) | Phase 1 | Treatment |
| NCT01782976 | A Phase II Trial of Cilengitide Plus Bevacizumab in Patients With Recurrent Glioblastoma | Glioblastoma | Phase 2 | Treatment |
| NCT00121238 | Phase II Evaluation of EMD 121974 (NSC 707544, Cilengitide) in Patients With Non-Metastatic Androgen Independent Prostate Cancer | Recurrent Prostate Cancer; Stage I Prostate Cancer; Stage IIA Prostate Cancer; Stage IIB Prostate Cancer; Stage III Prostate Cancer | Phase 2 | Treatment |
| NCT00093964 | A Multicenter, Open-label, Randomized, Uncontrolled, Phase IIa Trial in Subjects With Recurrent Glioblastoma Multiforme to Investigate the Clinical Activity, Safety, and Tolerability of Cilengitide (EMD 121,974) Administered as a Single Agent. | Glioblastoma Multiforme | Phase 2 | Treatment |
More clinical information is obtained from ClinicalTrials.gov.