Tecemotide
DRACPC ID DRACPC0058
Active Ingredients Tecemotide
Description A liposome-encapsulated peptide vaccine consisting of a synthetic peptide derived from the mucin 1 (MUC-1) antigen with potential antineoplastic activity. Upon vaccination, MUC-1 peptide vaccine may stimulate the host immune system to mount a cytotoxic T lymphocyte (CTL) response against MUC-1-expressing tumor cells, resulting in growth inhibition. MUC-1 antigen is a high-molecular-weight transmembrane glycoprotein that is overexpressed on the cell surfaces of many epithelial tumor cells as well as on the cell surfaces of some B-cell lymphoma cells and multiple myeloma cells.
Synonyms Emepepimut-S; BLP-25; BLP-25 Liposomal Vaccine; BLP25 Liposome Vaccine; BP1-7-KLH; EMD 531444; L-BLP25; Stimuvax; Glycine, L-seryl-L-threonyl-L-alanyl-L-prolyl-L-prolyl-L-alanyl-L-histidylglycyl-L-valyl-L-threonyl-L-seryl-L-alanyl-L-prolyl-L-alpha-aspartyl-L-threonyl-L-arginyl-L-prolyl-L-alanyl-L-prolylglycyl-L-seryl-L-threonyl-L-alanyl-L-prolyl-L-prolyl-N6-(1-oxohexadecyl)-L-lysyl-; Tecemotide
Type Biotech
Disease Prostate Cancer, Non-Small-Cell Lung
Classification
Peptide and derivative Vaccine
Structure Information
Molecular Formula C124H203N33O38
Molecular Weight 2764.1
Active Sequence STAPPAHGVTSAPDTRPAPGSTAPPXG
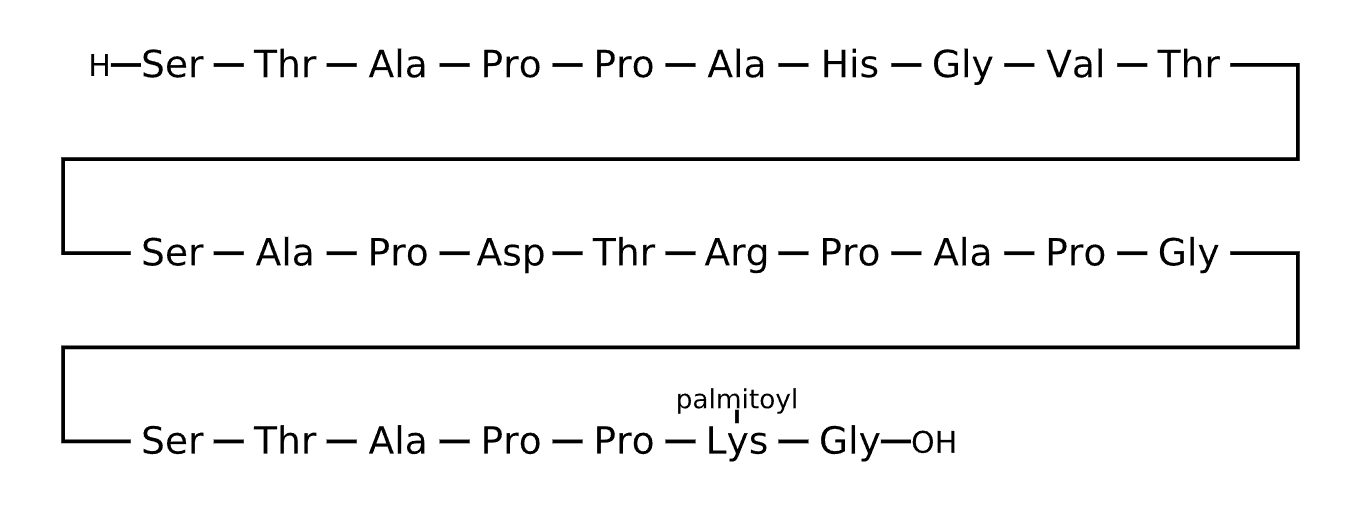
Sequence Length 27
Modification X=Lys(palmitoyl)
Structure
IUPAC Name Not available
InChI Not available
InChI_Key Not available
SMILES Not available
External Codes
PubChem CID 16208013
DrugBank Accession Number DB12568
NCI Thesaurus Code C2195
UNII Q7Y026G2CX GSRS
CAS 221214-84-2
Drug approval
Drug indication
Tecemotide has been used in trials studying the treatment of Prostate Cancer and Carcinoma, Non-Small-Cell Lung.
The drug is not approved.
| ClinicalTrials.gov Identifier | Title | Condition or disease | Phase | Purpose |
|---|---|---|---|---|
| NCT01423760 | An Open-label Trial to Collect Long-term Data on Subjects Following Participation in Previous EMD 531444 (L-BLP25 or BLP25 Liposome Vaccine) Clinical Trials | Non-Small Cell Lung Cancer; Multiple Myeloma | Not Applicable | Treatment |
| NCT01496131 | A Randomized Phase II Study of Tecemotide in Combination With Standard Androgen Deprivation Therapy and Radiation Therapy for Untreated, Intermediate and High Risk Prostate Cancer Patients | Prostate Cancer | Phase 2 | Treatment |
| NCT00157196 | A Multi-center, Non-randomized, Open Label Safety Study of BLP25 Liposome Vaccine (L-BLP25) in Non-Small Cell Lung Cancer (NSCLC) Patients With Unresectable Stage III Disease | Carcinoma, Non-Small-Cell Lung; Lung Neoplasms | Phase 2 | Treatment |
| NCT01507103 | A Multi-center, Randomized, Open-label, Mechanism of Action Trial on the Biological Effects of the Therapeutic Cancer Vaccine Stimuvax® (L-BLP25) in Rectal Cancer Subjects Undergoing Neoadjuvant Chemoradiotherapy | Rectal Cancer | Phase 2 | Treatment |
| NCT00157209 | A Multicenter Phase IIb Randomised, Controlled Study of BLP25 Liposome Vaccine for Active Specific Immunotherapy of Non-Small Cell Lung Cancer | Lung Neoplasms; Carcinoma, Non-Small-Cell Lung | Phase 2 | Treatment |
More clinical information is obtained from ClinicalTrials.gov.