Vapreotide
DRACPC ID DRACPC0060
Active Ingredients Vapreotide
Description A synthetic cyclic octapeptide analogue of somatostatin with direct and indirect antitumor effects. Vapreotide binds to somatostatin receptors (SSTR), specifically SSTR-2 and to SSTR-5 with a lesser affinity, in the similar behaviors as other octapeptide somatostatin analogues. Like octreotide, this agent has direct and indirect antitumor effects via inhibiting the release of growth hormone and other peptides that regulate release of insulin, gastrointestinal hormones. Furthermore, vapreotide may also be useful for inducing hemostasis in cases of acute hemorrhage of the upper gastrointestinal tract.
Synonyms BMY-41606; Docrised; RC-160; Vapreotide
Type Small Molecule
Disease Pancreatic Cancer
Classification
Somatostatin analogs Peptide and derivative Cyclic Hormone and analogue
Structure Information
Molecular Formula C57H70N12O9S2
Molecular Weight 1131.4
Active Sequence FCYWKVCW
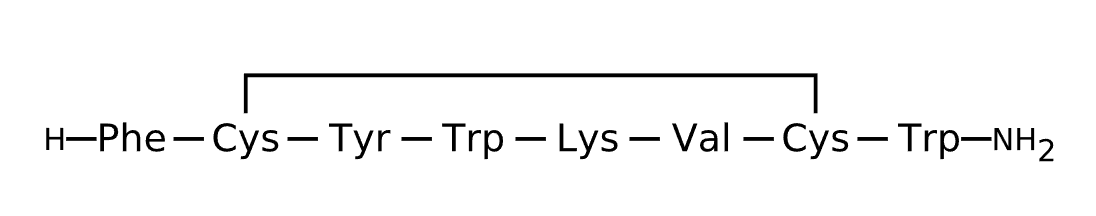
Sequence Length 8
Modification N-terminal NH2
IUPAC Name 10-(4-aminobutyl)-N-[1-amino-3-(1H-indol-3-yl)-1-oxopropan-2-yl]-19-[(2-amino-3-phenylpropanoyl)amino]-16-[(4-hydroxyphenyl)methyl]-13-(1H-indol-3-ylmethyl)-6,9,12,15,18-pentaoxo-7-propan-2-yl-1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carboxamide
InChI InChI=1S/C57H70N12O9S2/c1-32(2)49-57(78)68-48(55(76)64-44(50(60)71)26-35-28-61-41-16-8-6-14-38(35)41)31-80-79-30-47(67-51(72)40(59)24-33-12-4-3-5-13-33)56(77)65-45(25-34-19-21-37(70)22-20-34)53(74)66-46(27-36-29-62-42-17-9-7-15-39(36)42)54(75)63-43(52(73)69-49)18-10-11-23-58/h3-9,12-17,19-22,28-29,32,40,43-49,61-62,70H,10-11,18,23-27,30-31,58-59H2,1-2H3,(H2,60,71)(H,63,75)(H,64,76)(H,65,77)(H,66,74)(H,67,72)(H,68,78)(H,69,73)/t40-,43+,44+,45+,46-,47+,48+,49+/m1/s1
InChI_Key SWXOGPJRIDTIRL-DOUNNPEJSA-N
SMILES CC([C@@H]1NC([C@@H](NC([C@H](NC([C@@H](NC([C@H](CSSC[C@H](NC1=O)C(N[C@H](C(N)=O)CC2=CNC3=CC=CC=C23)=O)NC([C@@H](CC4=CC=CC=C4)N)=O)=O)CC5=CC=C(C=C5)O)=O)CC6=CNC7=CC=CC=C67)=O)CCCCN)=O)C
External Codes
PubChem CID 71306
DrugBank Accession Number DB04894
NCI Thesaurus Code C1429
UNII 2PK59M9GFF GSRS
CAS 103222-11-3
Drug approval
Drug indication
For the treatment of esophageal variceal bleeding in patients with cirrhotic liver disease and has also shown efficacy in the treatment of patients with AIDS-related diarrhea. It was being studied for the treatment of cancer.
The drug is not approved.
| ClinicalTrials.gov Identifier | Title | Condition or disease | Phase | Purpose |
|---|---|---|---|---|
| NCT00014651 | Vapreotide in Pancreas Surgery: A Double-Blind, Placebo-Controlled, Randomized Study of Vapreotide to Prevent Post-Surgical Complications in Patients Undergoing Elective Pancreatic Resection Grant Application Title: Vapreotide to Prevent Complications of Pancreatic Resection | Pancreatic Cancer; Perioperative/Postoperative Complications | Phase 3 | Supportive Care |
| NCT00331188 | The Early Use of Sanvar® With Endoscopic Treatment for the Control of Acute Variceal Bleeding Due to Portal Hypertension | Esophageal Varices; Portal Hypertension; Gastric Varices; Esophageal Bleeding | Phase 3 | Treatment |
More clinical information is obtained from ClinicalTrials.gov.