ABT-510
DRACPC ID DRACPC0061
Active Ingredients ABT-510
Description A synthetic peptide that mimics the anti-angiogenic activity of the endogenous protein thrombospondin-1 (TSP-1). ABT-510 inhibits the actions of several pro-angiogenic growth factors important to tumor neovascularization; these pro-angiogenic growth factors include vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF)), hepatocyte growth factor (HGF), and interleukin 8 (IL-8).
Synonyms TSP-1 Mimetic ABT-510; L-Prolinamide, N-acetyl-N-methylglycylglycyl-L-valyl-D-alloisoleucyl-L-threonyl-L-norvalyl-L-isoleucyl-L-arginyl-N-ethyl-; NAc-Sar-Gly-Val-(d-allo-Ile)-Thr-Nva-Ile-Arg-ProNEt; TSP-1-mimetic Peptide ABT-510; ABT-510
Type Small Molecule
Disease Lymphoma, Melanoma
Classification
Peptide and derivative Antiangiogenesis agent
Structure Information
Molecular Formula C46H83N13O11
Molecular Weight 994.2
Active Sequence XGVxTXIRP
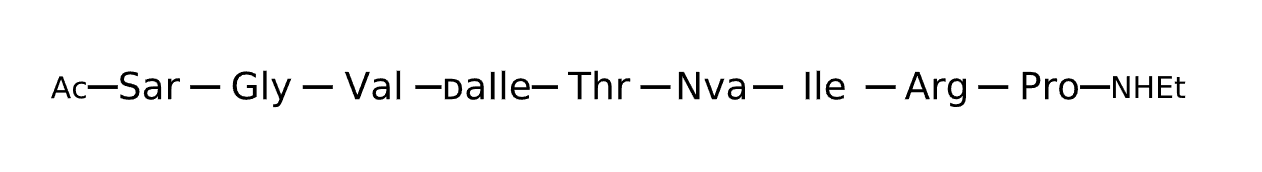
Sequence Length 9
Modification X(1)=Sar, x=D-aIle, X(6)=Nva, C-terminal Ac, N-terminal NHEt
IUPAC Name (2S)-1-[(2S)-2-[[(2S,3S)-2-[[(2S)-2-[[(2S,3R)-2-[[(2R,3S)-2-[[(2S)-2-[[2-[[2-[acetyl(methyl)amino]acetyl]amino]acetyl]amino]-3-methylbutanoyl]amino]-3-methylpentanoyl]amino]-3-hydroxybutanoyl]amino]pentanoyl]amino]-3-methylpentanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]-N-ethylpyrrolidine-2-carboxamide
InChI InChI=1S/C46H83N13O11/c1-12-18-30(39(64)55-36(26(7)13-2)42(67)53-31(19-16-21-50-46(47)48)45(70)59-22-17-20-32(59)40(65)49-15-4)52-44(69)38(28(9)60)57-43(68)37(27(8)14-3)56-41(66)35(25(5)6)54-33(62)23-51-34(63)24-58(11)29(10)61/h25-28,30-32,35-38,60H,12-24H2,1-11H3,(H,49,65)(H,51,63)(H,52,69)(H,53,67)(H,54,62)(H,55,64)(H,56,66)(H,57,68)(H4,47,48,50)/t26-,27-,28+,30-,31-,32-,35-,36-,37+,38-/m0/s1
InChI_Key RIWLPSIAFBLILR-WVNGMBSFSA-N
SMILES O=C([C@H]1N(C([C@@H](NC([C@@H](NC([C@@H](NC([C@@H](NC([C@H](NC([C@@H](NC(CNC(CN(C(C)=O)C)=O)=O)C(C)C)=O)[C@@H](C)CC)=O)[C@H](O)C)=O)CCC)=O)[C@@H](C)CC)=O)CCC/N=C(N)\N)=O)CCC1)NCC
External Codes
PubChem CID 6918562
DrugBank Accession Number DB05434
NCI Thesaurus Code C37447
UNII CRR8E37XOB GSRS
CAS 442526-86-5
Drug approval
Drug indication
Investigated for use/treatment in lymphoma (unspecified), melanoma, and solid tumors.
The drug is not approved.
| ClinicalTrials.gov Identifier | Title | Condition or disease | Phase | Purpose |
|---|---|---|---|---|
| NCT00113334 | A Phase 1b/2 Study of ABT-510 (Thrombospondin Analogue) in Patients With Advanced Head and Neck Cancer | Head and Neck Cancer | Phase 1/2 | Treatment |
| NCT00584883 | A Phase I Study of ABT 510 and Concurrent Temozolomide and Radiotherapy for Patients With Newly Diagnosed Glioblastoma Multiforme | Brain Tumor | Phase 1 | Treatment |
| NCT00073125 | A Phase II Randomized Study Evaluating the Safety and Efficacy of ABT-510 in Subjects With Advanced Renal Cell Carcinoma | Carcinoma, Renal Cell | Phase 2 | Treatment |
| NCT00061672 | A Phase II Study Evaluating the Safety and Effectiveness of ABT-510 in Subjects With Refractory Lymphoma | Lymphoma, Non-Hodgkin; Hodgkin's Lymphoma | Phase 2 | Treatment |
| NCT00061659 | A Phase II Randomized Study Evaluating the Safety and Efficacy of ABT-510 in Subjects With Locally Advanced or Metastatic Soft Tissue Sarcoma | Sarcoma, Soft Tissue | Phase 2 | Treatment |
More clinical information is obtained from ClinicalTrials.gov.