Iseganan
DRACPC ID DRACPC0066
Active Ingredients Iseganan
Description A protegrin analogue containing 17 amino acids, which is effective for oral microorganisms.
Synonyms Antimicrobial peptide IB-367; IB 367; IB367; IB-367; Iseganan
Type Biotech
Disease Head and Neck Cancer
Classification
Peptide and derivative Cyclic
Structure Information
Molecular Formula C78H126N30O18S4
Molecular Weight 1900.3
Active Sequence RGGLCYCRGRFCVCVGR
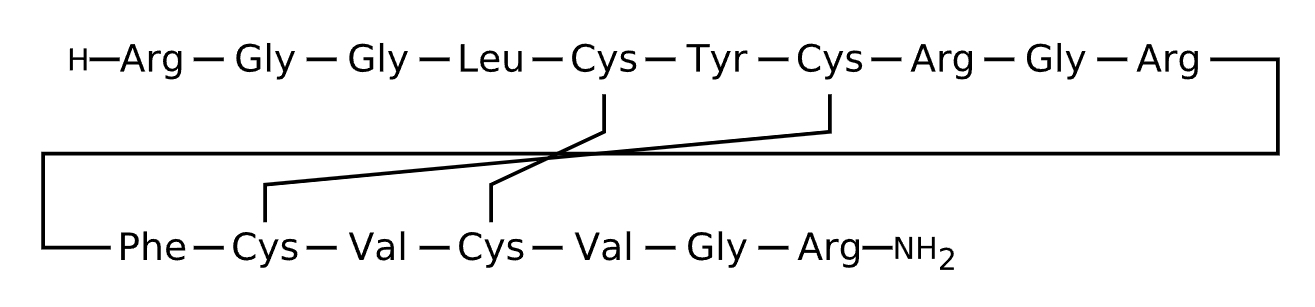
Sequence Length 17
Modification N-terminal NH2
IUPAC Name (1R,4S,7R,12R,15S,18R,21S,24S,30S)-N-[(2S)-1-[[2-[[(2S)-1-amino-5-carbamimidamido-1-oxopentan-2-yl]amino]-2-oxoethyl]amino]-3-methyl-1-oxobutan-2-yl]-7-[[(2S)-2-[[2-[[2-[[(2S)-2-amino-5-carbamimidamidopentanoyl]amino]acetyl]amino]acetyl]amino]-4-methylpentanoyl]amino]-21-benzyl-24,30-bis(3-carbamimidamidopropyl)-4-[(4-hydroxyphenyl)methyl]-3,6,14,17,20,23,26,29,32-nonaoxo-15-propan-2-yl-9,10,34,35-tetrathia-2,5,13,16,19,22,25,28,31-nonazabicyclo[16.14.4]hexatriacontane-12-carboxamide
InChI InChI=1S/C78H126N30O18S4/c1-39(2)28-49(99-57(111)32-93-56(110)31-94-63(115)45(79)16-10-24-89-75(81)82)66(118)103-53-36-128-130-38-55(72(124)107-60(40(3)4)73(125)96-34-58(112)97-46(62(80)114)17-11-25-90-76(83)84)106-74(126)61(41(5)6)108-71(123)54-37-129-127-35-52(104-68(120)51(102-70(53)122)30-43-20-22-44(109)23-21-43)69(121)100-47(18-12-26-91-77(85)86)64(116)95-33-59(113)98-48(19-13-27-92-78(87)88)65(117)101-50(67(119)105-54)29-42-14-8-7-9-15-42/h7-9,14-15,20-23,39-41,45-55,60-61,109H,10-13,16-19,24-38,79H2,1-6H3,(H2,80,114)(H,93,110)(H,94,115)(H,95,116)(H,96,125)(H,97,112)(H,98,113)(H,99,111)(H,100,121)(H,101,117)(H,102,122)(H,103,118)(H,104,120)(H,105,119)(H,106,126)(H,107,124)(H,108,123)(H4,81,82,89)(H4,83,84,90)(H4,85,86,91)(H4,87,88,92)/t45-,46-,47-,48-,49-,50-,51-,52-,53-,54-,55-,60-,61-/m0/s1
InChI_Key GUCYBPFJNGVFEB-XELKFLSISA-N
SMILES CC(C[C@@H](C(N[C@H]1CSSC[C@H](NC([C@@H](NC([C@@H]2CSSC[C@@H](C(N[C@H](C(NCC(N[C@H](C(N[C@H](C(N2)=O)CC3=CC=CC=C3)=O)CCCNC(N)=N)=O)=O)CCCNC(N)=N)=O)NC([C@@H](NC1=O)CC4=CC=C(C=C4)O)=O)=O)C(C)C)=O)C(N[C@@H](C(C)C)C(NCC(N[C@H](C(N)=O)CCCNC(N)=N)=O)=O)=O)=O)NC(CNC(CNC([C@H](CCCNC(N)=N)N)=O)=O)=O)C
External Codes
PubChem CID 16131103
DrugBank Accession Number DB16010
NCI Thesaurus Code C174882
UNII Q9SAI36COS GSRS
CAS 257277-05-7
Drug approval
Drug indication
Investigated for use/treatment in Head and Neck Cancer.
The drug is not approved.
| ClinicalTrials.gov Identifier | Title | Condition or disease | Phase | Purpose |
|---|---|---|---|---|
| NCT00118781 | Randomized, Double-Blind, Placebo-Controlled, Multinational Phase 3 Trial Of Iseganan In Prevention Of Ventilator-Associated Pneumonia | Pneumonia | Phase 2/3 | Prevention |
| NCT00022373 | A Multinational, Multicenter, Double-Blind, Placebo-Controlled, Randomized, Phase III Clinical Trial to Determine the Efficacy and Safety of IB-367 Rinse in Reducing the Severity of Oral Mucositis in Patients Receiving Radiotherapy for Head and Neck Malignancy | Head and Neck Cancer; Oral Complications of Radiation Therapy; Radiation Toxicity | Phase 3 | Supportive Care |
More clinical information is obtained from ClinicalTrials.gov.