Mipsagargin
DRACPC ID DRACPC0069
Active Ingredients Mipsagargin
Description A soluble, thapsigargin prodrug containing the cytotoxic analog of thapsigargin, 8-O-(12Aminododecanoyl)-8-O debutanoylthapsigargin (12-ADT) linked, via a carboxyl group, to the targeting peptide containing aspartic acid with potential antineoplastic activity. Upon intravenous administration, mipsagargin targets prostate specific membrane antigen (PSMA), a type II membrane carboxypeptidase, which is overexpressed in prostate cancer cells and in the neovasculature of most solid tumors but not in normal blood vessels. Mipsagargin is subsequently converted, through hydrolysis, into the active cytotoxic analog of thapsigargin 12-ADT-Asp. 12-ADT binds to and blocks the Sarcoplasmic/Endoplasmic Reticulum Calcium ATPase (SERCA) pump, thereby increasing the concentration of cytosolic calcium which leads to an induction of apoptosis. By preventing nutrient supply to tumor cells, G-202 may be able to inhibit tumor growth. Compared to thapsigargin alone, thapsigargin prodrug G-202 is able to achieve higher concentrations of the active agents at the tumor site while avoiding systemic toxicity.
Synonyms G-202; Mipsagargin
Type Small Molecule
Disease Prostate Cancer, Advanced Solid Tumors, Glioblastoma Multiforme, Hepatocellular Carcinoma
Classification
Peptide and derivative
Structure Information
Molecular Formula C66H100N6O27
Molecular Weight 1409.5
Active Sequence XXXXE
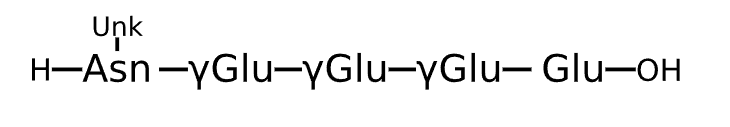
Sequence Length 5
Modification X(1)=Asn(Unk), X(2/3/4)=gGlu
IUPAC Name (2S)-2-[[(4S)-4-[[(4S)-4-[[(4S)-4-[[(2S)-4-[[12-[[(3S,3aR,4S,6S,6aR,7S,8S,9bS)-6-acetyloxy-3,3a-dihydroxy-3,6,9-trimethyl-8-[(Z)-2-methylbut-2-enoyl]oxy-7-octanoyloxy-2-oxo-4,5,6a,7,8,9b-hexahydroazuleno[4,5-b]furan-4-yl]oxy]-12-oxododecyl]amino]-2-amino-4-oxobutanoyl]amino]-4-carboxybutanoyl]amino]-4-carboxybutanoyl]amino]-4-carboxybutanoyl]amino]pentanedioic acid
InChI InChI=1S/C66H100N6O27/c1-8-10-11-17-20-24-51(81)96-55-53-52(37(4)54(55)97-62(91)36(3)9-2)56-66(94,65(7,93)63(92)98-56)44(35-64(53,6)99-38(5)73)95-50(80)23-21-18-15-13-12-14-16-19-22-33-68-48(77)34-39(67)57(82)72-43(61(89)90)27-31-47(76)70-41(59(85)86)25-29-45(74)69-40(58(83)84)26-30-46(75)71-42(60(87)88)28-32-49(78)79/h9,39-44,53-56,93-94H,8,10-35,67H2,1-7H3,(H,68,77)(H,69,74)(H,70,76)(H,71,75)(H,72,82)(H,78,79)(H,83,84)(H,85,86)(H,87,88)(H,89,90)/b36-9-/t39-,40-,41-,42-,43-,44-,53+,54-,55-,56-,64-,65+,66+/m0/s1
InChI_Key UPYNTAIBQVNPIH-ODMLWHIESA-N
SMILES C/C=C(C(O[C@H]1C(C)=C2[C@@H]3OC([C@@](O)([C@@]([C@H](C[C@](OC(C)=O)([C@H]2[C@@H]1OC(CCCCCCC)=O)C)OC(CCCCCCCCCCCNC(C[C@@H](C(N[C@H](C(O)=O)CCC(N[C@H](C(O)=O)CCC(N[C@H](C(O)=O)CCC(N[C@H](C(O)=O)CCC(O)=O)=O)=O)=O)=O)N)=O)=O)3O)C)=O)=O)/C
External Codes
PubChem CID 24772106
DrugBank Accession Number DB11813
NCI Thesaurus Code C90554
UNII Q032I35QMX GSRS
CAS 1245732-48-2
Drug approval
Drug indication
Mipsagargin has been used in trials studying the treatment of Prostate Cancer, Prostatic Neoplasms, Advanced Solid Tumors, Glioblastoma Multiforme, and Hepatocellular Carcinoma, among others.
The drug is not approved.
| ClinicalTrials.gov Identifier | Title | Condition or disease | Phase | Purpose |
|---|---|---|---|---|
| NCT01777594 | A Phase II, Multicenter, Single-Arm Study of G-202 (Mipsagargin) as Second-Line Therapy Following Sorafenib for Adult Patients With Progressive Advanced Hepatocellular Carcinoma | Advanced Adult Hepatocellular Carcinoma | Phase 2 | Treatment |
| NCT02067156 | An Open-Label, Single-Arm, Phase II Study to Evaluate the Efficacy, Safety and CNS Exposure of G-202 (Mipsagargin) in Patients With Recurrent or Progressive Glioblastoma | Glioblastoma Multiforme | Phase 2 | Treatment |
| NCT01056029 | An Open Label, Single-Arm, Dose-Escalation Phase 1 Study of G-202 (Mipsagargin) in Patients With Advanced Solid Tumors | Advanced Solid Tumors | Phase 1 | Treatment |
| NCT02607553 | G-202-006: An Open-Label, Single-Arm, Phase II Study to Evaluate the Safety and Activity of G-202 in Patients With Clear Cell Renal Cell Carcinoma That Expresses PSMA | Clear Cell Renal Cell Carcinoma | Phase 2 | Treatment |
| NCT02381236 | G-202-005: An Open-Label, Single-Arm, Phase II Study to Evaluate the Safety and Activity of G-202 Administered in the Neoadjuvant Setting Followed by Radical Prostatectomy in Patients With Adenocarcinoma of the Prostate | Prostatic Neoplasms | Phase 2 | Treatment |
More clinical information is obtained from ClinicalTrials.gov.