Ozarelix
DRACPC ID DRACPC0071
Active Ingredients Ozarelix
Description A highly modified, fourth generation linear decapeptide with gonadotropin-releasing hormone (GnRH or LHRH) antagonizing properties. Ozarelix competitively binds to and blocks the gonadotropin releasing hormone receptor in the anterior pituitary gland, thereby inhibiting the secretion and release of luteinizing hormone (LH) and follicle stimulating hormone (FSH). In males, the inhibition of LH secretion prevents the release of testosterone. As a result, this may relieve symptoms associated with hormonally dependent disease states such as hormone-dependent prostate cancer.
Synonyms SPI-153; D 63153; D-63 153; D63 153; D63153; LHRH antagonist SPI-153; Ozarelix
Type Small Molecule
Disease Prostate Cancer, Benign Prostatic Hypertrophy, Benign Prostatic Hyperplasia
Classification
GnRH/LHRH antagonist Peptide and derivative
Structure Information
Molecular Formula C72H96ClN17O14
Molecular Weight 1459.1
Active Sequence xxxSXxXRPa
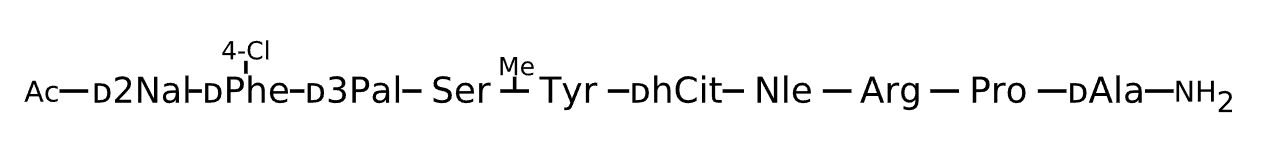
Sequence Length 10
Modification x(1)=D-2Nal, x(2)=D-Phe(4-Cl), x(3)=D-3Pal, X(5)=N(Me)Tyr, x(6)=D-hCit, X(7)=Nle, C-terminal Ac, N-terminal NH2
IUPAC Name (2S)-1-[(2S)-2-[[(2S)-2-[[(2R)-2-[[(2S)-2-[[(2S)-2-[[(2R)-2-[[(2R)-2-[[(2R)-2-acetamido-3-naphthalen-2-ylpropanoyl]amino]-3-(4-chlorophenyl)propanoyl]amino]-3-pyridin-3-ylpropanoyl]amino]-3-hydroxypropanoyl]-methylamino]-3-(4-hydroxyphenyl)propanoyl]amino]-6-(carbamoylamino)hexanoyl]amino]hexanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]-N-[(2R)-1-amino-1-oxopropan-2-yl]pyrrolidine-2-carboxamide
InChI InChI=1S/C72H96ClN17O14/c1-5-6-17-52(63(96)85-54(19-12-33-79-71(75)76)70(103)90-34-13-20-59(90)67(100)81-42(2)61(74)94)83-62(95)53(18-9-10-32-80-72(77)104)84-68(101)60(39-45-24-29-51(93)30-25-45)89(4)69(102)58(41-91)88-66(99)57(38-47-14-11-31-78-40-47)87-65(98)56(36-44-22-27-50(73)28-23-44)86-64(97)55(82-43(3)92)37-46-21-26-48-15-7-8-16-49(48)35-46/h7-8,11,14-16,21-31,35,40,42,52-60,91,93H,5-6,9-10,12-13,17-20,32-34,36-39,41H2,1-4H3,(H2,74,94)(H,81,100)(H,82,92)(H,83,95)(H,84,101)(H,85,96)(H,86,97)(H,87,98)(H,88,99)(H4,75,76,79)(H3,77,80,104)/t42-,52+,53-,54+,55-,56-,57-,58+,59+,60+/m1/s1
InChI_Key KATZUZNTRINHDT-HALMFYTRSA-N
SMILES [H]N([C@H](C(N([C@H](C(N[C@@H](C(N[C@H](C(N[C@H](C(N1CCC[C@H]1C(N([C@@H](C(N)=O)C)[H])=O)=O)CCCNC(N)=N)=O)CCCC)=O)CCCCNC(N)=O)=O)CC2=CC=C(C=C2)O)C)=O)CO)C([C@H](NC([C@H](NC([C@H](NC(C)=O)CC3=CC4=CC=CC=C4C=C3)=O)CC5=CC=C(C=C5)Cl)=O)CC6=CN=CC=C6)=O
External Codes
PubChem CID 25080293
DrugBank Accession Number DB12581
NCI Thesaurus Code C95214
UNII Q1IF8M2YL3 GSRS
CAS 295350-45-7
Drug approval
Drug indication
Ozarelix has been used in trials studying the treatment of Prostate Cancer, Benign Prostatic Hypertrophy, and Benign Prostatic Hyperplasia (BPH).
The drug is not approved.
| ClinicalTrials.gov Identifier | Title | Condition or disease | Phase | Purpose |
|---|---|---|---|---|
| NCT00427219 | A Randomized, Double-Blind, Placebo-Controlled Trial of the Safety and Efficacy of Ozarelix, in Patients With Lower Urinary Tract Symptoms(LUTS) Due to Benign Prostatic Hypertrophy (BPH) | Benign Prostatic Hypertrophy | Phase 2 | Treatment |
| NCT01252693 | Phase 2 Study Assessing the Safety and Efficacy of a Monthly Dosing Regimen of Ozarelix Versus Goserelin Depot (Zoladex®) in Men With Prostate Cancer | Prostate Cancer | Phase 2 | Treatment |
| NCT00743184 | A Multi-Center, Randomized, Double-Blind, Placebo-Controlled Trial of the Safety and Efficacy of Ozarelix, in Patients With Lower Urinary Tract Symptoms (LUTS) Due to Benign Prostatic Hyperplasia (BPH) | Benign Prostatic Hyperplasia (BPH); Lower Urinary Tract Symptoms (LUTS) | Phase 2 | Treatment |
More clinical information is obtained from ClinicalTrials.gov.