Talotrexin
DRACPC ID DRACPC0074
Active Ingredients Talotrexin
Description An antimetabolite analogue of aminopterin with potential antineoplastic activity. As a folate antagonist, talotrexin binds to and inhibits the function of dihydrofolate reductase, resulting in the inhibition of folate metabolism, DNA synthesis, and cell division. Hydrosoluble, talotrexin is actively transported into cells by the reduced folate carrier (RFC) and, therefore, is unlikely to be associated with P-glycoprotein-mediated multidrug resistance.
Synonyms PT523; N(alpha)-(4-Amino-4-deoxypteroyl)-N(delta)-hemiphthaloyl-L-ornithine; Talopterin; Talotrexin
Type Small Molecule
Disease Lung cancer, Leukemia
Classification
Folate antagonist Peptide and derivative
Structure Information
Molecular Formula C27H27N9O6
Molecular Weight 573.6
Active Sequence Not available
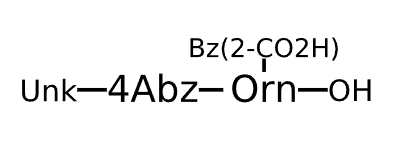
Sequence Length Not available
Modification Not available
IUPAC Name 2-[[(4S)-4-carboxy-4-[[4-[(2,4-diaminopteridin-6-yl)methylamino]benzoyl]amino]butyl]carbamoyl]benzoic acid
InChI InChI=1S/C27H27N9O6/c28-21-20-22(36-27(29)35-21)32-13-16(33-20)12-31-15-9-7-14(8-10-15)23(37)34-19(26(41)42)6-3-11-30-24(38)17-4-1-2-5-18(17)25(39)40/h1-2,4-5,7-10,13,19,31H,3,6,11-12H2,(H,30,38)(H,34,37)(H,39,40)(H,41,42)(H4,28,29,32,35,36)
InChI_Key NYQPLPNEESYGNO-UHFFFAOYSA-N
SMILES NC1=NC(N)=C2N=C(C=NC2=N1)CNC3=CC=C(C=C3)C(NC(C(O)=O)CCCNC(C4=C(C=CC=C4)C(O)=O)=O)=O
External Codes
PubChem CID 130731
DrugBank Accession Number DB06178
NCI Thesaurus Code C29341
UNII A8E516A20K GSRS
CAS 113857-87-7
Drug approval
Drug indication
Investigated for use/treatment in solid tumors, lung cancer, leukemia (unspecified), and leukemia (lymphoid).
The drug is not approved.
| ClinicalTrials.gov Identifier | Title | Condition or disease | Phase | Purpose |
|---|---|---|---|---|
| NCT00458744 | A Phase I Study Of Talotrexin (PT-523) In Children And Adolescents With Recurrent Solid Tumors Or Recurrent/Refractory Leukemias | Brain and Central Nervous System Tumors; Leukemia; Lymphoma; Unspecified Childhood Solid Tumor, Protocol Specific | Phase 1 | Treatment |
| NCT00098514 | A Phase I Study Of PT523 In Patients With Solid Tumors | Unspecified Adult Solid Tumor, Protocol Specific | Phase 1 | Treatment |
| NCT00112060 | A Phase I/II, Open-Label, Multicenter Study of Single Agent PT-523 in the Treatment of Relapsed or Refractory Non-Small Cell Lung Cancer (NSCLC) | Non-Small-Cell Lung Carcinoma | Phase 1/2 | Treatment |
More clinical information is obtained from ClinicalTrials.gov.