Teverelix
DRACPC ID DRACPC0075
Active Ingredients Teverelix
Description A synthetic decapeptide and antagonist of the naturally occurring gonadotropin-releasing hormone (GnRH), with potential hormone production inhibitory and antineoplastic activities. Upon administration, teverelix directly competes with GnRH for receptor binding in the anterior pituitary gland, thereby inhibiting GnRH receptor signaling. This inhibits the secretion and release of luteinizing hormone (LH) and follicle stimulating hormone (FSH). In males, the inhibition of LH secretion prevents the release of testosterone. Since testosterone is required to sustain prostate growth, reducing testosterone levels may inhibit hormone-dependent prostate cancer cell proliferation. In females, this prevents the production of estrogen by the ovaries and may relieve symptoms from sex-hormone dependent diseases.
Synonyms ANB019; EP 24332; Ac-D-Nal-D-cpa-D-pal-ser-tyr-D-hci-leu-lys(ipr)-pro-D-ala-NH2; D-Alaninamide, N-acetyl-3-(2-naphthalenyl)-D-alanyl-4-chloro-D-phenylalanyl-3-(3-pyridinyl)-D-alanyl-L-seryl-L-tyrosyl-N6-(aminocarbonyl)-D-lysyl-L-leucyl-N6-(1-methylethyl)-L-lysyl-L-prolyl-; Teverelix
Type Small Molecule
Disease Prostate cancer, Prostatic hyperplasia, Endometriosis
Classification
GnRH antagonist Peptide and derivative
Structure Information
Molecular Formula C74H100ClN15O14
Molecular Weight 1459.1
Active Sequence xxxSYxLXPa
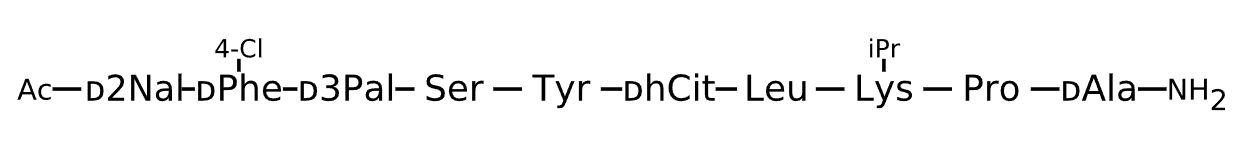
Sequence Length 10
Modification x(1)=D-2Nal, x(2)=D-Phe(4-Cl), x(3)=D-3Pal, x(6)=D-hCit, X(8)=Lys(iPr), C-terminal Ac, N-terminal NH2
IUPAC Name (2S)-1-[(2S)-2-[[(2S)-2-[[(2R)-2-[[(2S)-2-[[(2S)-2-[[(2R)-2-[[(2R)-2-[[(2R)-2-acetamido-3-naphthalen-2-ylpropanoyl]amino]-3-(4-chlorophenyl)propanoyl]amino]-3-pyridin-3-ylpropanoyl]amino]-3-hydroxypropanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-6-(carbamoylamino)hexanoyl]amino]-4-methylpentanoyl]amino]-6-(propan-2-ylamino)hexanoyl]-N-[(2R)-1-amino-1-oxopropan-2-yl]pyrrolidine-2-carboxamide
InChI InChI=1S/C74H100ClN15O14/c1-43(2)35-57(66(96)84-56(19-10-11-32-79-44(3)4)73(103)90-34-14-20-63(90)72(102)81-45(5)64(76)94)85-65(95)55(18-9-12-33-80-74(77)104)83-68(98)59(38-48-24-29-54(93)30-25-48)88-71(101)62(42-91)89-70(100)61(40-50-15-13-31-78-41-50)87-69(99)60(37-47-22-27-53(75)28-23-47)86-67(97)58(82-46(6)92)39-49-21-26-51-16-7-8-17-52(51)36-49/h7-8,13,15-17,21-31,36,41,43-45,55-63,79,91,93H,9-12,14,18-20,32-35,37-40,42H2,1-6H3,(H2,76,94)(H,81,102)(H,82,92)(H,83,98)(H,84,96)(H,85,95)(H,86,97)(H,87,99)(H,88,101)(H,89,100)(H3,77,80,104)/t45-,55-,56+,57+,58-,59+,60-,61-,62?,63+/m1/s1
InChI_Key NOENHWMKHNSHGX-DGWYIYCFSA-N
SMILES OC(C=C1)=CC=C1C[C@H](NC(C(NC([C@H](NC([C@H](NC([C@H](NC(C)=O)CC2=CC3=C(C=C2)C=CC=C3)=O)CC4=CC=C(Cl)C=C4)=O)CC5=CC=CN=C5)=O)CO)=O)C(N[C@H](CCCCNC(N)=O)C(N[C@H](C(N[C@@H](CCCCNC(C)C)C(N6[C@@H](CCC6)C(N[C@H](C)C(N)=O)=O)=O)=O)CC(C)C)=O)=O
External Codes
PubChem CID 16135076
DrugBank Accession Number DB05624
NCI Thesaurus Code C132277
UNII D19V7048JK GSRS
CAS 151272-78-5
Drug approval
Drug indication
Investigated for use/treatment in benign prostatic hyperplasia, endometriosis, and prostate cancer.
The drug is not approved.
| ClinicalTrials.gov Identifier | Title | Condition or disease | Phase | Purpose |
|---|---|---|---|---|
| NCT04693507 | An Adaptive Phase 2, Open-Label, Multicentre Study Investigating the Pharmacokinetics, Pharmacodynamics, Efficacy and Safety of Teverelix Trifluoroacetate, a GnRH Antagonist, in Participants With Advanced Prostate Cancer | Prostatic Adenoma | Phase 2 | Treatment |
| NCT03781947 | A Phase I, Open-label, Single Centre Study Investigating the PK, Safety and PD of a Single Dose of Teverelix TFA, a GnRH Antagonist, Via s.c. or i.m. Route of Administration in Healthy Male Volunteers | Healthy | Phase 1 | Basic Science |
More clinical information is obtained from ClinicalTrials.gov.